Corrosion threatens metal materials like stainless steel and aluminum, undermining their durability, functionality, and appearance. Whether you’re dealing with industrial machinery, kitchen appliances, or outdoor structures, understanding how and why these metals corrode is essential for protecting your investments. This comprehensive guide explores the science behind corrosion and provides practical solutions for long-term protection.
Introduction to Corrosion

Corrosion is the slow degradation of materials, especially metals, resulting from their interaction with environmental elements such as oxygen, moisture, or chemicals. This natural phenomenon changes the surface or structure of materials, gradually weakening them over time.
Key Facts About Corrosion
- Corrosion causes approximately $2.5 trillion in economic losses annually worldwide
- Both stainless steel and aluminum are corrosion-resistant but not completely immune
- Proper treatment and maintenance are essential for maintaining durability and appearance
Definition of Corrosion
Corrosion represents the natural, slow degradation of metals through chemical or electrochemical interactions between the metal and its environment. This process typically involves oxidation, where metals react with substances like:
- Oxygen: Leading to oxide formation
- Moisture: Accelerating electrochemical reactions
- Acids and salts: Creating aggressive corrosive environments
- Industrial pollutants: Adding chemical contaminants
Why Study Corrosion?
Understanding corrosion is crucial for:
- Preserving valuable resources and infrastructure
- Improving safety in industrial applications
- Reducing economic losses through prevention
- Developing sustainable operational practices
- Contributing to environmental protection
Material Overview: Stainless Steel vs. Aluminum

| Property | Stainless Steel | Aluminum |
|---|---|---|
| Primary Composition | Iron, Chromium, Nickel | Pure Aluminum with various alloys |
| Weight | Heavy (3x heavier than aluminum) | Lightweight |
| Corrosion Protection | Chromium oxide passive layer | Natural aluminum oxide layer |
| Primary Applications | Construction, transport, kitchenware | Aerospace, automotive, packaging, electrical |
| Strength | High strength and durability | Good strength-to-weight ratio |
Mechanisms of Corrosion
Types of Corrosion
Electrochemical Corrosion
Occurs in moist conditions through anodic and cathodic reactions. Most common form affecting both materials.
Pitting Corrosion
Creates small holes or abrasions, often caused by chloride ions in marine environments.
Galvanic Corrosion
Occurs when dissimilar metals are electrically coupled in a conductive environment.
Crevice Corrosion
Develops in confined spaces where oxygen access is limited, common in stainless steel.
What is Galvanic Corrosion?
Critical Concern: When two different metals are in electrical contact within a conductive environment, galvanic corrosion creates a galvanic cell. One metal acts as an anode (corrodes faster) while the other becomes the cathode (protected). The severity depends on:
- Position of metals in the galvanic series
- Environmental conductivity
- Anode-to-cathode area ratio
Corrosion in Stainless Steel and Aluminum
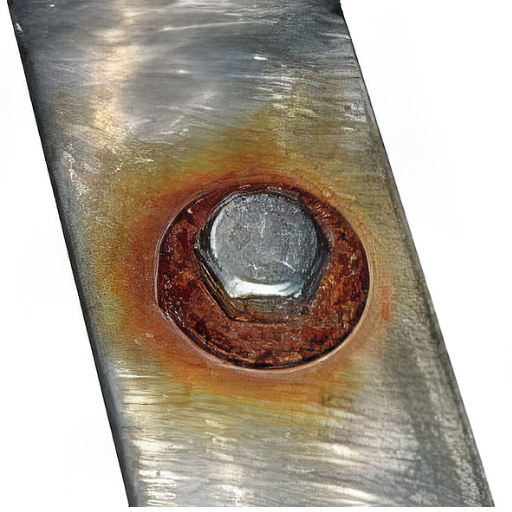
Stainless Steel Corrosion
Stainless steel’s corrosion resistance comes from a passive chromium oxide barrier. However, this protection can be compromised in:
- Chloride-rich environments: Seawater, deicing salts
- High-temperature conditions: Above 1000°F (538°C)
- Low-oxygen environments: Crevices and confined spaces
- Acidic conditions: pH below 4
Aluminum Corrosion
Aluminum forms a natural protective oxide layer, but vulnerabilities include:
- Galvanic corrosion: Contact with more noble metals
- Acidic environments: pH below 4
- Alkaline conditions: pH above 8.5
- Chloride exposure: Salt-rich environments
Factors Influencing Corrosion Rates
| Factor Category | Specific Elements | Impact on Corrosion |
|---|---|---|
| Environmental | Humidity, Temperature, pH, Salinity | Higher values accelerate corrosion rates |
| Material Properties | Alloy composition, Surface finish, Coatings | Better alloys and treatments reduce corrosion |
| Mechanical Factors | Stress, Abrasion, Surface defects | Physical damage accelerates local corrosion |
| Chemical Exposure | Pollutants, Industrial emissions, Chemicals | Aggressive agents increase corrosion rates |
Corrosion Resistance Comparison
Performance Analysis
Stainless Steel (Grade 316): Superior corrosion resistance, especially in marine environments due to molybdenum content. Excellent for harsh chemical processing applications.
Aluminum Alloys (6061, 7075): Good corrosion resistance with lightweight advantages. Requires additional protection (anodizing, coatings) for extreme environments.
Preventing Galvanic Corrosion
Material Selection
Choose metals close together on the galvanic series. The smaller the potential difference, the lower the corrosion risk.
Protective Coatings
Apply non-conductive coatings to prevent electrical contact. Regular inspection ensures coating integrity.
Insulation Methods
Use insulating materials (rubber, plastic washers) between dissimilar metals to prevent direct contact.
Cathodic Protection
Implement sacrificial anodes or impressed current systems to redirect corrosion to disposable materials.
Environmental Control
Minimize moisture and electrolyte exposure through proper drainage and environmental controls.
Design Optimization
Avoid designs that trap electrolytes. Implement proper drainage and ventilation systems.
Strategies for Using Aluminum and Stainless Steel Together

Best Practices
- Use Barrier Materials: Insert rubber or plastic washers between metals
- Select Compatible Grades: Choose Grade 316 stainless steel or duplex alloys
- Control Environment: Maintain low humidity and good ventilation
- Choose Proper Fasteners: Use compatible or non-metallic fastening materials
- Implement Monitoring: Install real-time corrosion monitoring systems
- Regular Maintenance: Schedule routine inspections and preventive care
Role of Sacrificial Anodes
Sacrificial anodes made from zinc, magnesium, or aluminum protect structural metals by corroding preferentially. This cost-effective method is widely used in:
- Marine applications
- Underground pipelines
- Water heater tanks
- Bridge structures
Maintenance Practices

Regular Inspection and Monitoring
Modern maintenance incorporates AI-based tools and predictive analytics to:
- Identify wear patterns with unprecedented accuracy
- Enable predictive maintenance scheduling
- Prevent critical failures before they occur
- Optimize maintenance resources and timing
Cleaning Techniques
| Material | Recommended Method | Avoid |
|---|---|---|
| Stainless Steel | Soft cloth with mild soap and water, immediate drying | Abrasive cleaners, steel wool, prolonged moisture exposure |
| Aluminum | Gentle detergents, microfiber cloths, thorough rinsing | Harsh chemicals that damage oxide layer, abrasive materials |
Protective Coatings and Treatments
Advanced protective solutions include:
- Nanotechnology-based coatings: Ultra-thin protective barriers
- Eco-friendly treatments: Environmentally sustainable options
- Smart coatings: Self-healing and adaptive protection systems
- Anodizing: Enhanced oxide layer protection for aluminum
References
-
Corrosion of Stainless Steel and Aluminum Alloys in Fuming Nitric Acid – University of Michigan – Discusses the corrosion behavior of aluminum and stainless steel in specific environments.
-
Galvanic Corrosion of Aluminum Coupled to Stainless Steel – University of Hawaii – Explores galvanic corrosion between aluminum and stainless steel in various environmental conditions.
-
Determining the Evolution of Galvanic Corrosion – Harvard ADS – Examines the interaction between aluminum and stainless steel, focusing on their passive oxide films and galvanic corrosion.
Frequently Asked Questions (FAQ)
Define galvanic corrosion between stainless steel and aluminum!
Galvanic corrosion occurs when two dissimilar metals, e.g., stainless steel and aluminum, are in electrical contact with an electrolyte present, leading to accelerated corrosion of one of the metals. In this case, aluminum is more likely to be the one corroding, being lower on the galvanic scale than stainless steel.
How can I prevent galvanic corrosion between stainless steel and aluminum?
To prevent galvanic corrosion, insulating materials can be introduced between stainless steel and aluminum. Secondly, coatings can be applied or fasteners bonded from the same metal.
How do aluminum and stainless steel compare with regard to corrosion resistance?
Aluminum has generally good corrosion resistance by nature because of its oxide layer. On the other hand, stainless steel is corrosion-resistant due to its chromium oxide layer. However, under some conditions, such as in a saltwater environment, aluminum may corrode faster than stainless steel.
Can stainless steel fasteners be used with aluminum parts?
Yes, fasteners of stainless steel can be used with aluminum parts, but it’s important to do so carefully to avoid galvanic corrosion. One way to prevent corrosion is by using stainless steel screws and isolating them from direct contact with the aluminum.
What are the effects of galvanic corrosion on aluminum and stainless steel?
There is an accelerated corrosion rate of aluminum parts due to the effect of galvanic corrosion, resulting in the aluminum alloy interface junction between steel and aluminum weakening and possible failure. Such applications must be avoided when both metals are exposed to a harsh environment, such as saltwater.
Is it safe to use stainless steel and aluminum in construction?
Although stainless steel and aluminum can be used together in construction, measures have to be taken to ensure safe use. Isolating the two metals and providing them with proper coatings will prevent galvanic corrosion and ensure that the materials will last longer.
Which aluminum alloy is best to use with stainless steel?
When using aluminum alongside stainless steel, aluminum alloys such as 6061 or 7075 are generally preferred because of their strength and corrosion resistance. These alloys help minimize the chances of galvanic corrosion if placed next to stainless steel parts.
How does salt water affect the corrosion of aluminum and stainless steel?
Salt water quickens the corrosion process for both aluminum and stainless steel. In a saltwater environment, aluminum is a high risk of galvanic corrosion while in contact with stainless steel, leading to very rapid degradation of the aluminum parts.