Austenite represents one of the most crucial phases in steel metallurgy, serving as the cornerstone for developing high-performance stainless steel alloys. Whether you’re a materials engineer, metalworking professional, or simply curious about the science behind everyday products, understanding austenite’s role reveals why certain materials excel in demanding applications.
Understanding Austenite: The Foundation
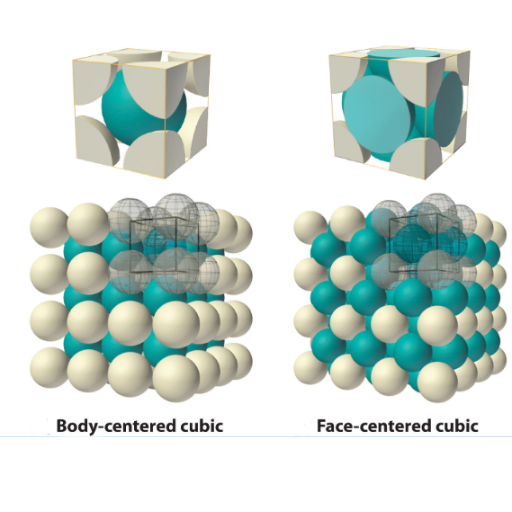
Austenite is the face-centered cubic (FCC) crystal structure of iron, also known as gamma iron (γ-iron). This non-magnetic allotrope of iron forms at elevated temperatures and can dissolve significantly more carbon than other iron phases, making it essential for steel production and heat treatment processes.
Key Characteristics of Austenite
- Crystal Structure: Face-centered cubic (FCC) arrangement
- Temperature Range: Stable above 723°C (1333°F)
- Carbon Solubility: Up to 2.0 weight percent
- Magnetic Properties: Non-magnetic
- Density: Higher than ferrite due to FCC packing
Formation and Phase Transformation
How Austenite Forms
Atomic Rearrangement: Iron atoms shift from BCC to FCC crystal structure
Carbon Dissolution: Increased carbon solubility in the FCC structure
Stabilization: Alloying elements (Ni, Mn) can stabilize austenite at room temperature
Heat Treatment Processes
Heat treatment processes leverage austenite formation to control final material properties:
- Austenitization: Heating to form austenite
- Quenching: Rapid cooling to form martensite
- Normalizing: Air cooling for pearlite formation
- Annealing: Controlled cooling for desired microstructure
Properties of Austenite

| Property Category | Characteristic | Significance |
|---|---|---|
| Mechanical Properties | High ductility and toughness | Excellent formability and impact resistance |
| Thermal Properties | Lower thermal conductivity than ferrite | Better heat retention and thermal barriers |
| Corrosion Resistance | Excellent with proper alloying | Forms protective oxide layers |
| Magnetic Properties | Non-magnetic (paramagnetic) | Useful in electronic and precision applications |
| Work Hardening | Significant strain hardening | Strength increases with cold working |
Types of Steel Structures Comparison
| Steel Type | Primary Elements | Key Properties | Common Applications |
|---|---|---|---|
| Austenitic | Fe-Cr-Ni (18-20% Cr, 8-12% Ni) | Excellent corrosion resistance, high ductility, non-magnetic | Food processing, chemical equipment, medical devices |
| Martensitic | Fe-Cr (11-18% Cr, low Ni) | High hardness and strength, magnetic | Cutlery, surgical instruments, turbine blades |
| Ferritic | Fe-Cr (12-30% Cr, no Ni) | Good corrosion resistance, magnetic, lower cost | Automotive exhaust, decorative trim, appliances |
Industrial Applications of Austenitic Stainless Steel

Storage tanks, conveyor systems, and processing equipment benefit from austenitic steel’s hygienic properties and resistance to acidic/alkaline environments.
Reactors, heat exchangers, and piping systems utilize austenitic steel’s resistance to corrosive chemicals and high-temperature stability.
Surgical instruments, implants, and hospital equipment leverage the biocompatibility and sterilization compatibility of austenitic grades.
Structural elements, cladding, and decorative features utilize the durability and aesthetic appeal of austenitic stainless steel.
Exhaust systems, fuel tanks, and aircraft components benefit from high strength-to-weight ratio and temperature resistance.
Power plant components, nuclear equipment, and oil/gas pipelines rely on austenitic steel’s high-temperature strength and corrosion resistance.
High-Temperature Applications
Critical High-Temperature Uses
- Power Generation: Boiler tubes, heat exchangers, and turbine components operating at temperatures up to 1,093°C (2,000°F)
- Aerospace Industry: Aircraft engine components and exhaust systems handling extreme thermal cycling
- Chemical Processing: Reactors and distillation equipment operating under high temperature and corrosive conditions
- Industrial Furnaces: Components for annealing, hardening, and heat treatment processes
- Automotive Exhaust: Catalytic converters and manifolds managing varying temperature environments
Common Austenitic Stainless Steel Grades

| Grade | Composition | Key Features | Primary Applications |
|---|---|---|---|
| 304 | 18% Cr, 8% Ni | General-purpose, good corrosion resistance | Kitchen equipment, food processing, architecture |
| 316 | 18% Cr, 10% Ni, 2% Mo | Enhanced corrosion resistance, chloride resistance | Marine environments, chemical processing, medical |
| 316L | Low carbon version of 316 | Improved weldability, reduced carbide precipitation | Surgical implants, pharmaceutical equipment |
| 310 | 25% Cr, 20% Ni | Excellent high-temperature oxidation resistance | Furnace components, heat exchangers |
Everyday Products Containing Austenite
Household Applications
- Kitchen Equipment: Sinks, countertops, appliances, and cookware
- Architectural Elements: Door handles, window frames, railings, and decorative panels
- Transportation: Automotive exhaust systems, train components, and marine hardware
- Tools and Hardware: Fasteners, springs, and precision instruments
References
-
Austenitizing in Steels – Colorado School of Mines – Discusses the high-temperature, face-centered cubic form of iron and its stability in the iron-carbon phase diagram.
-
Materials – Princeton University – Explains the transition of austenite to other phases during cooling and its role in material properties.
-
Austenite and Ferrite Grain Size Evolution – U.S. Department of Energy (OSTI) – Analyzes the relationship between austenite microstructure and ferrite grain size in plain carbon steel.
Frequently Asked Questions (FAQ)
what is austenite and what are its properties?
Austenite is a verb of steel which is above the critical eutectoid temperature and has also a face-centered cubic (fcc) crystal structure of iron. Such type of iron allows higher carbon solubility as compared to ferrite, own bearing capable of forming a solid solution with alloying elements. Among many properties of austenite are the most significant strength, toughness, and corrosion resistance especially when dealing with stainless steels.
How is the austenitic form developed in a material?
If there is a temperature increase above a critical temperature in iron or solid solution, the iron atoms shift and start accumulating into either a body-centered cubic or a face-centered cubic form which is called austenite. This phenomenon is usually observed in the process of heat treatment which entails slight manipulation in the heating and cooling processes alongside changing phases and properties of the material and encouraging the conversion of alpha iron into austenite.
What is inherent/not inherent in martensitic transformation of austenite?
In metallurgy, quenching is one of the several ways which results in formation of martensite, a hard and brittle phase, from austenite any time quenching is carried out, with the exception that, some of the austenite does not transform to martensite on quenching but rather remains as austenite in a transformed product known as retained austenite. The relative amounts of martensite to such retained austenite also help control the mechanical properties that are being developed in the steel.
What stainless steel can undergo a martensitic or austenic phase transformation?
Particularly,basing on austenitic class of stainless steel – a very remarkable example is the SS 304 grade. Due to the high content of the elements such as nickel and chromium, these steels are sturdy and workable because these elements help to form and hold upon a substantial amount of austenitic structure.
What is austenite and under what conditions does it form?
To begin with, it is well known that the crystal structures of most metals change from one to another with change of temperature, and in Iron, austenite is present at temperatures above the critical eutectoid temperature. Carbon or alloy content stabilizes austenite, but stability may also be due to other factors. Austenite may also be mainly produced at high temperatures – above critical transitions in stead of lower temperatures – in carbon steels, and can be transformed .
what is the importance of Sir William Chandler Roberts-Austen associating with AUSTENITE?
Sir William Chandler Roberts-Austen, who was also called as ‘Sir W. A. Chandler’, was a British metallurgist known for the powerful advances of some phases of steel in particular austenite. This is because physical metallurgy science performed by this researcher focused on development of iron and steel alloys at elevated temperatures, which has a relationship with modern methods of steel working and heat processing.
What is the effect of the Austenite structure on these materials?
One of the main factors that determine the mechanical properties of metals, particularly high-carbon and stainless steels, is the microstructure’s austenitic phase. Formation of austenite helps in improving ductility, toughness, and also stress corrosion cracking resistance. Transformation of its structure through heat enhances the properties of the steel however these transformations are catered for specific applications.
What is the criteria for distinguishing austenite from the lime in concrete in contrast to cementite?
Austenite is present in steels and, in particular interacts with the two other phases contained therein i.e. ferrite and cementite steel. Over the iron-carbon diagram austenite allows the formation of lamella ferrite and cementite at low temperatures. Such eutectic transformations prove crucial in enhancing the necessary metallurgical qualities of plain carbon steel.