Steel, one of the most versatile and widely used materials in the world, owes its diverse applications to the distinct microstructures that define its properties. Among these, ferrite and austenite steels stand out as two fundamental categories, each with unique characteristics that make them suitable for specific industrial uses. But what sets these two types of steel apart, and why does it matter? This article aims to demystify the differences between ferrite and austenite steel, exploring their composition, mechanical properties, and optimal applications. Whether you’re an engineer, materials scientist, or simply curious about the science behind steel, this comprehensive guide will provide the critical insights needed to better understand these essential materials.
What Is Ferrite in Steel?

Ferrite in steel refers to a body-centered cubic (BCC) phase of iron that is relatively soft and ductile. It forms the microstructure of steel at lower temperatures and contains a small amount of dissolved carbon, typically less than 0.02%. Ferrite contributes to the steel’s malleability and strength, making it an essential component in various applications requiring flexibility and toughness. It is predominant in low-carbon and mild steels, where its properties enhance workability and weldability.
Understanding the Crystal Structure of Ferrite
Ferrite possesses a body-centered cubic (BCC) crystal structure, which is a key determinant of its physical and mechanical properties. The BCC structure consists of one atom at each corner of a cube and a single atom at its center. This arrangement provides ferrite with a relatively low atomic packing factor of 0.68, contributing to its lower density compared to other materials with different structures, such as face-centered cubic (FCC). Due to this configuration, ferrite remains soft and ductile, yet it exhibits sufficient strength for applications demanding malleability. Recent advancements in materials science emphasize the role of lattice distortions in influencing the behavior of ferrite under varying stress and temperature conditions, further enhancing its utility in diverse industrial uses.
The Role of Carbon in Ferritic Stainless Steel
- Strength Enhancement: Carbon contributes to the strengthening of ferritic stainless steel through solid-solution strengthening, where carbon atoms distort the crystal lattice, impeding dislocation motion and increasing overall material strength.
- Impact on Ductility: While carbon enhances strength, excessive carbon content can reduce the ductility of ferritic stainless steel, leading to the necessity for precise control during alloy design and processing.
- Corrosion Resistance: Lower carbon levels are critical for maintaining the excellent corrosion resistance of ferritic stainless steel by minimizing the formation of chromium carbides that could deplete chromium from the matrix.
- Weldability: Carbon levels significantly influence the weldability of ferritic stainless steels, with low carbon content aiding in the prevention of sensitization that can lead to intergranular corrosion in welded sections.
- Thermal Stability: Carbon affects the thermal stability of ferritic stainless steel, particularly in terms of its phase stability and resistance to high-temperature degradation during prolonged service.
Exploring the Magnetic Properties of Ferrite
The magnetic properties of ferrite are largely determined by its crystal structure, chemical composition, and microstructural characteristics. Ferritic stainless steels, composed primarily of iron and chromium, exhibit ferromagnetic behavior due to their body-centered cubic (BCC) structure. This structure allows unpaired electrons to align, resulting in strong magnetic attraction.
Ferrite’s intrinsic magnetic permeability is relatively high, making it suitable for electromagnetic applications such as transformers and magnetic cores. However, factors such as grain size, temperature, and alloying elements significantly influence its magnetism. For example, increasing the chromium content can reduce magnetic saturation due to the dilution of iron’s ferromagnetic properties.
One key characteristic of ferritic stainless steel is its low coercivity, meaning it requires minimal energy to demagnetize. This property is advantageous for applications requiring soft magnetic materials, as it ensures efficiency during alternating magnetization cycles. Understanding these magnetic features is essential for tailoring ferritic alloys to specific industrial and engineering needs.
What Is Austenite in Steel?
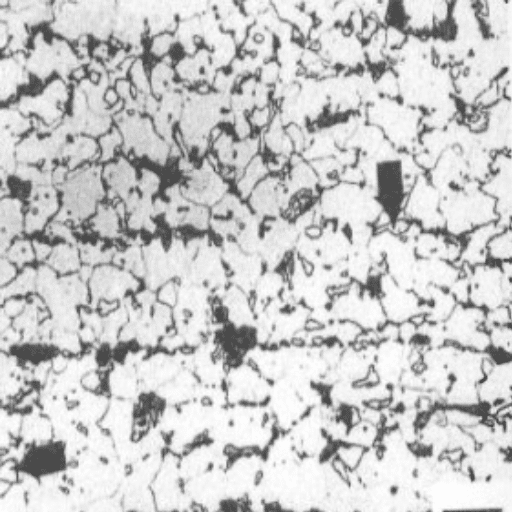
Austenite, also known as gamma-phase iron (γ-Fe), is a face-centered cubic (FCC) structure of iron or steel that exists at elevated temperatures, typically above 912°C (1,674°F) in pure iron. This phase is stabilized in steel through the addition of alloying elements such as nickel, manganese, and nitrogen, which expand the temperature range in which austenite is thermodynamically stable.
A key feature of austenite is its ability to dissolve significantly more carbon compared to ferrite, up to approximately 2.14% by weight at 1148°C (2098°F). This characteristic makes austenite critical in processes such as carburizing and in the production of high-strength steels. The high solubility of carbon also facilitates the formation of other microstructures, such as martensite, when austenitic steel is rapidly quenched.
Austenitic stainless steels, distinguished by their high chromium (approximately 16-26%) and nickel (often 6-22%) content, are corrosion-resistant and non-magnetic in most conditions. These steels are widely used in industries such as chemical processing, aerospace, and construction due to their excellent mechanical properties, weldability, and resistance to oxidation. The study and manipulation of austenite’s properties play an instrumental role in developing advanced steel grades tailored for specific engineering applications.
Exploring the Crystalline Structure of Austenite
Austenite possesses a face-centered cubic (FCC) crystalline structure, which contributes significantly to its mechanical and physical properties. This structure is highly symmetrical, providing excellent ductility and the ability to absorb significant deformation without fracturing. The FCC arrangement offers a higher atomic packing density compared to body-centered cubic (BCC) structures, resulting in superior strength and toughness.
The stability of austenite’s crystalline structure is influenced by its chemical composition and temperature. Elements such as nickel, manganese, and nitrogen serve as austenite stabilizers, maintaining the FCC structure even at lower temperatures. Conversely, elements like chromium and molybdenum can promote transformation into other phases, such as martensite or ferrite, under specific conditions. This phase transformation capability is critical in producing steels with tailored mechanical and corrosion resistance properties, further expanding the utility of austenitic alloys across diverse industries.
Understanding the crystalline behavior of austenite allows metallurgists to optimize alloy design for applications demanding high performance under extreme conditions, including high-temperature environments and aggressive chemical exposure.
Impact of Carbon Solubility in Austenitic Stainless Steel
Carbon solubility significantly influences the mechanical properties, phase stability, and corrosion resistance of austenitic stainless steels. At elevated temperatures, carbon readily dissolves in the austenitic phase, contributing to solid solution strengthening. However, excessive carbon levels can lead to the precipitation of chromium carbide (Cr23C6) along grain boundaries during cooling or heat treatment. This carbide formation reduces the chromium content in the surrounding matrix, resulting in localized depletion zones susceptible to intergranular corrosion—a phenomenon known as sensitization.
Key technical parameters related to carbon solubility in austenitic stainless steels include:
- Maximum Carbon Content (per ASTM standards): Typically ≤ 0.03 wt% for low-carbon grades (e.g., 304L, 316L) to minimize carbide precipitation and enhance weldability.
- Solution Annealing Temperature: Commonly in the range of 1,040–1,120°C to dissolve carbides and retain austenite phase integrity during heat treatment.
- Cooling Rate: Rapid cooling (e.g., water quenching) is essential to suppress carbide precipitation and preserve corrosion resistance.
- Chromium Content: Minimum 18 wt% chromium to ensure the formation of a stable passive oxide layer and to counteract the effects of sensitization.
Effective control of carbon solubility through alloy composition adjustments and precise heat treatment practices is necessary to maintain optimal performance characteristics in demanding applications.
Understanding Austenite’s Non-Magnetic Nature
Austenite’s non-magnetic behavior arises from its face-centered cubic (FCC) crystal structure, which lacks the unpaired electron spin alignment required for ferromagnetism. Unlike ferrite, which has a body-centered cubic (BCC) structure, austenite does not form magnetic domains capable of producing strong magnetic fields. The presence of elements like nickel and the stabilization of the FCC phase at specific temperature ranges further prevent the formation of magnetic properties within austenitic stainless steels. Additionally, alloying elements that promote the austenite phase play a key role in maintaining its non-magnetic nature. Consequently, this characteristic makes austenitic stainless steels ideal for applications requiring non-magnetic and corrosion-resistant properties.
The Difference Between Ferrite and Austenite in Steel
Ferrite and austenite are two distinct microstructural forms of steel, each with unique properties and phase behavior. Ferrite is a body-centered cubic (BCC) phase, known for its magnetic properties, relatively low strength, and high ductility. It is the stable phase of iron at room temperature and typically forms in low-carbon steels. Austenite, on the other hand, is a face-centered cubic (FCC) phase that exists at higher temperatures or is stabilized by alloying elements such as nickel and manganese. Unlike ferrite, austenite is non-magnetic, has higher strength, and can maintain its structure in a wide temperature range when properly alloyed. The primary difference lies in their crystal structures and magnetic behaviors, which dictate their applications in varying industrial contexts.
Comparing Mechanical Properties of Ferrite and Austenite
Ferrite and austenite differ in their mechanical properties including hardness, tensile strength, ductility, and toughness, each influenced by their crystal structure and composition. Below is a concise comparison of their key mechanical properties:
|
Property |
Ferrite |
Austenite |
|---|---|---|
|
Hardness |
Low |
Higher |
|
Strength |
Moderate |
High |
|
Ductility |
High |
Very High |
|
Toughness |
Moderate |
Excellent |
|
Magnetism |
Magnetic |
Non-Magnetic |
|
Thermal Exp. |
Low |
Higher |
How Temperature Affects Ferrite and Austenite Phases
Temperature plays a vital role in the stability and transformation of ferrite and austenite phases in steel. Ferrite, characterized by a body-centered cubic (BCC) structure, is stable at lower temperatures, typically below 912°C. Above this temperature, ferrite transitions into austenite, which possesses a face-centered cubic (FCC) structure, due to increased atomic mobility that facilitates the rearrangement of the crystal lattice.
Austenite remains stable in a temperature range between 912°C and 1394°C. Above 1394°C, austenite transforms into delta ferrite, a high-temperature phase with a BCC structure. The exact transformation temperatures may vary depending on the chemical composition, particularly the carbon content and alloying elements such as nickel or chromium. For example:
- Pure iron (Fe): Ferrite-to-Austenite transformation at 912°C, Austenite-to-Delta Ferrite at 1394°C.
- Carbon steel (0.8% C): Transformation temperatures shift slightly due to carbon’s effect on the phase boundaries.
This sensitivity to temperature is critical in industrial applications such as heat treatment, welding, and phase-controlled manufacturing, where maintaining precise thermal conditions directly impacts mechanical properties like hardness, toughness, and ductility.
Examining Corrosion Resistance Differences
Corrosion resistance in metals is primarily influenced by composition, surface treatment, and environmental conditions. Stainless steels, due to their chromium content (minimum 10.5%), form a passive oxide layer, granting superior resistance, especially in oxidizing environments. Alloyed steels, such as those containing nickel, molybdenum, or manganese, enhance corrosion performance in reducing or acidic conditions. Carbon steels, lacking such alloying elements, are highly vulnerable to rust when exposed to moisture and oxygen unless coated or treated. Environmental factors like chloride presence (e.g., in marine settings) or high humidity amplify corrosion rates. Hence, material selection for critical applications hinges on analyzing corrosive exposure, alloy composition, and required service life.
What Is Ferritic Stainless Steel?

Ferritic stainless steel is a category of stainless steel primarily composed of chromium (typically ranging between 10.5% and 30%) with little to no nickel content. This alloy exhibits a body-centered cubic (BCC) crystal structure, which makes it magnetic and distinct from austenitic stainless steels. Ferritic stainless steels are renowned for their superior resistance to stress corrosion cracking and oxidation, making them well-suited for applications in environments with varying temperatures and moderate corrosive conditions. They also provide good thermal conductivity and resistance to scaling at high temperatures, making them a preferred choice in industries such as automotive exhaust systems, heat exchangers, and kitchen equipment.
Recent advancements have improved the weldability and toughness of ferritic grades, although their ductility may still be lower compared to austenitic types. The absence of nickel also makes ferritic stainless steels a cost-effective option in situations where nickel prices are volatile. Overall, their composition and properties make ferritic stainless steels versatile and widely used, especially where strength, corrosion resistance, and cost-efficiency are prioritized.
Composition and Microstructure of Ferritic Steels
Ferritic stainless steels are primarily composed of iron and chromium, with chromium content typically ranging between 10.5% and 30%. The absence of significant amounts of nickel is a distinguishing feature, which contributes to their cost-effectiveness compared to austenitic steels. Depending on the grade, ferritic steels may also include small amounts of other alloying elements, such as molybdenum (up to 4%), titanium, niobium, and aluminum, which enhance specific properties like corrosion resistance and grain refinement.
The microstructure of ferritic stainless steels is predominantly body-centered cubic (BCC), which confers high strength but limits ductility at lower temperatures compared to the face-centered cubic (FCC) microstructure of austenitic steels. This BCC structure is stable across a wide range of temperatures, ensuring excellent oxidation resistance and scaling resistance at high temperatures, often up to 750°C (1,382°F). Notably, the lack of phase transformation during heating and cooling contributes to their dimensional stability.
Key Technical Parameters:
- Chromium Content: 10.5%–30% (primary element for corrosion resistance)
- Molybdenum (optional): Up to 4% for improved pitting resistance.
- Thermal Conductivity: Higher than austenitic steels, making them suitable for heat exchangers.
- Coefficient of Thermal Expansion: Lower than austenitic grades, reducing thermal distortion in applications.
- Yield Strength: Typically ranging from 240 MPa to 450 MPa depending on the specific grade.
- Operating Temperature Range: Effective up to approximately 750°C for high-temperature applications.
These characteristics make ferritic stainless steels ideal for applications in automotive exhaust systems, kitchen equipment, boilers, and architectural structures where durability and resistance to oxidation are critical. Their unique combination of affordability, strength, and corrosion resistance ensures their continued relevance across numerous industrial sectors.
Applications and Mechanical Properties
- Automotive Exhaust Systems: Due to their high-temperature oxidation resistance and cost-effectiveness, ferritic stainless steels are widely used in exhaust system components, including manifolds and catalytic converters.
- Industrial Boilers and Heat Exchangers: Their excellent thermal conductivity and resistance to stress corrosion cracking make them suitable for handling high-temperature and high-pressure environments.
- Kitchen Equipment and Appliances: The corrosion resistance and durability of ferritic stainless steel ensure long-lasting performance in cooking utensils, sinks, and dishwashers.
- Architectural Cladding and Structures: Their aesthetic appeal, combined with excellent weathering properties, makes them a popular choice for modern architectural designs and exterior applications.
- Energy Applications: Ferritic stainless steels are employed in renewable energy systems, including solar panels, owing to their strength and resistance to thermal stress.
Understanding Austenitic Stainless Steel

Austenitic stainless steels represent the most versatile and widely used category of stainless steels, characterized by their high corrosion resistance, excellent formability, and superior weldability. This category primarily contains chromium and nickel as alloying elements, often supplemented with molybdenum to enhance resistance to chloride-induced pitting and crevice corrosion. Unlike ferritic grades, austenitic stainless steels maintain their strength and ductility across a broad temperature range, making them suitable for both cryogenic and high-temperature applications.
Commonly included in the 300-series (e.g., 304, 316), austenitic stainless steels are employed across various industries such as food processing, chemical processing, and medical instrumentation due to their non-magnetic nature and hygienic properties. Their high chromium content ensures the formation of a stable passive oxide layer, offering prolonged durability even in highly aggressive environments. This combination of properties makes them an indispensable material for critical applications where reliability and resistance to environmental factors are crucial.
Role of Nickel in Austenitic Steels
- Stabilizing the Austenitic Phase: Nickel plays a crucial role in stabilizing the austenitic crystal structure at room temperature, ensuring the steel retains its toughness and ductility under various conditions.
- Enhanced Corrosion Resistance: By incorporating nickel, austenitic steels exhibit improved resistance to corrosion in both acidic and alkaline environments, making them suitable for demanding industrial applications.
- Improved Weldability: Nickel reduces the tendency for cracking during welding processes, ensuring structural integrity after fabrication and facilitating complex assembly operations.
- Low-Temperature Toughness: Nickel contributes to increased toughness at sub-zero temperatures, making austenitic steels reliable for cryogenic applications.
- Enhanced Formability: The addition of nickel ensures better formability, allowing austenitic steels to be easily shaped, drawn, or rolled into desired geometries without losing mechanical properties.
Why Austenitic Steels are Highly Ductile?
Austenitic steels are highly ductile primarily due to their unique face-centered cubic (FCC) crystal structure. This structure provides a high number of slip systems, allowing the material to deform plastically under stress without fracturing. Additionally, the high nickel content enhances their mechanical versatility by stabilizing the FCC phase, even at varying temperatures. The absence of a ductile-to-brittle transition temperature in these steels further ensures consistent ductility across a wide range of conditions. These attributes make austenitic steels particularly suitable for demanding applications where formability and toughness are critical.
Reference Sources
- Difference between Ferrite and Austenite Steel – Nickel Alloys Online
- Difference between Ferrite and Austenite Steel material – Oshwin
- The Difference Between Austenitic and Ferritic Stainless Steel – Monroe Engineering
- Ferritic vs. Martensitic vs. Austenitic – AATProd
- Understanding Ferritic vs Austenitic vs Martensitic Stainless Steel – Arc Machines
Frequently Asked Questions (FAQs)
Q: What is the primary difference between austenite and ferrite in steel?
A: The primary difference between austenite and ferrite in steel lies in their metallurgical structure and the solubility of carbon. Austenite has a face-centered cubic crystal structure, which allows for higher solubility of carbon, while ferrite has a body-centered cubic structure with lower carbon solubility. This leads to differences in mechanical properties and applications.
Q: How does the solubility of carbon in austenite compare to ferrite?
A: The solubility of carbon in austenite is significantly higher than in ferrite. Austenite can dissolve up to 2.0% carbon at high temperatures due to its face-centered cubic structure, whereas ferrite can only dissolve about 0.02% carbon due to its body-centered cubic structure.
Q: Why is ferrite magnetic while austenite is not?
A: Ferrite is magnetic because of its body-centered cubic crystal structure, which allows the alignment of magnetic moments. In contrast, austenite’s face-centered cubic structure does not support such alignment, making it non-magnetic.
Q: What role does carbon play in the formation of ferrite and austenite?
A: Carbon plays a critical role in the formation of ferrite and austenite by influencing their crystalline structures and properties. In ferrite, the low solubility of carbon results in a harder phase, while in austenite, the higher solubility allows for more ductile and tough properties.
Q: How do different types of stainless steel relate to austenitic and ferritic structures?
A: Stainless steels are categorized based on their metallurgical structure. Austenitic stainless steels are known for their high chromium and nickel content, promoting austenite, while ferritic stainless steels contain more chromium and less nickel, promoting the formation of ferrite.
Q: What is the significance of the ferrite number in steel?
A: The ferrite number indicates the amount of ferrite present in stainless steel welds. It helps assess the balance between austenite and ferrite to ensure desirable mechanical properties and corrosion resistance in the final weldment.
Q: How does the presence of ferrite affect the mechanical properties of steel?
A: The presence of ferrite tends to make steel stronger and more magnetic but less ductile compared to austenite. Ferrite crystals contribute to increased strength due to their different crystalline and metallurgical structure.
Q: What factors promote the formation of ferrite over austenite in steel?
A: Factors that promote the formation of ferrite over austenite include higher chromium content, lower nickel content, and lower carbon levels. These elements affect the cubic crystal structure, favoring the body-centered cubic structure of ferrite.
Q: Why does ferrite have a higher density compared to austenite?
A: Ferrite has a higher density compared to austenite because its body-centered cubic structure is more tightly packed than the face-centered cubic structure of austenite, resulting in a denser arrangement of iron atoms.
Q: How does the amount of carbon affect the formation of pearlite in relation to ferrite?
A: The amount of carbon significantly affects the formation of pearlite, which is a mixture of ferrite and cementite. Higher carbon content promotes the formation of pearlite by increasing the amount of carbon available to form cementite alongside ferrite crystals.