Galvanic corrosion is a costly and potentially dangerous process that occurs when dissimilar metals come into contact in the presence of an electrolyte. This electrochemical reaction can compromise structural integrity, increase maintenance costs, and lead to catastrophic failures in marine, construction, and infrastructure applications.
Whether you’re working in shipbuilding, construction, or infrastructure management, understanding and preventing galvanic corrosion is essential for project safety and longevity. This comprehensive guide will provide you with proven strategies to protect your investments and extend equipment life.
What is Galvanic Corrosion?
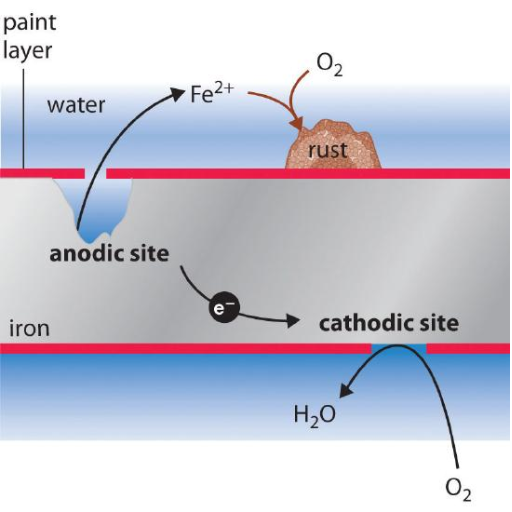
Galvanic corrosion, also known as bimetallic corrosion, occurs when two dissimilar metals are electrically connected in the presence of an electrolyte (such as saltwater, moisture, or even humid air). This creates a galvanic cell where:
- The anode (less noble metal) loses electrons and corrodes rapidly
- The cathode (more noble metal) gains electrons and remains protected
- The electrolyte allows ionic flow between the metals
Understanding the Electrochemical Process
Key Point: The greater the difference in electrochemical potential between two metals, the faster the corrosion rate of the anodic material. This is why material selection is crucial in preventing galvanic corrosion.
Common Metal Combinations and Risk Factors
| Metal Combination | Risk Level | Common Applications | Prevention Strategy |
|---|---|---|---|
| Steel + Aluminum | High | Automotive, Marine | Insulation, compatible fasteners |
| Steel + Stainless Steel | Medium | Pipelines, Structures | Protective coatings, isolation |
| Aluminum + Copper | Very High | Electrical systems | Avoid direct contact, use barriers |
| Iron + Zinc | Beneficial | Galvanizing | Intentional sacrificial protection |
| Titanium + Steel | Medium-High | Aerospace | Insulative gaskets, coatings |
Environmental Factors That Accelerate Corrosion
Primary Contributing Conditions
- Marine environments – Saltwater provides highly conductive electrolyte
- High humidity – Promotes moisture accumulation and ionic activity
- Industrial atmospheres – Chemical pollutants increase corrosivity
- Temperature fluctuations – Thermal cycling promotes moisture condensation
- pH extremes – Acidic or highly alkaline conditions accelerate reactions
⚠️ Critical Warning: Galvanic corrosion failures have led to structural collapses in marine infrastructure, pipeline leaks causing environmental damage, and aircraft component failures. Proper prevention is not optional—it’s essential for safety.
Real-World Impact and Consequences

Industry Examples of Galvanic Corrosion Failures
🚢 Marine Industry
Ship hulls using mixed steel and aluminum components have experienced rapid deterioration in seawater, leading to increased maintenance costs and vessel downtime. Some cases have resulted in structural failures requiring emergency repairs.
🔧 Pipeline Systems
Mixed carbon steel and stainless steel components in high-salinity soil environments have caused pipeline leaks, environmental contamination, and massive cleanup costs.
✈️ Aerospace Industry
Aluminum aircraft parts fastened with dissimilar metal bolts have shown accelerated corrosion in atmospheric conditions, requiring intensive monitoring and replacement programs.
Comprehensive Prevention Strategies
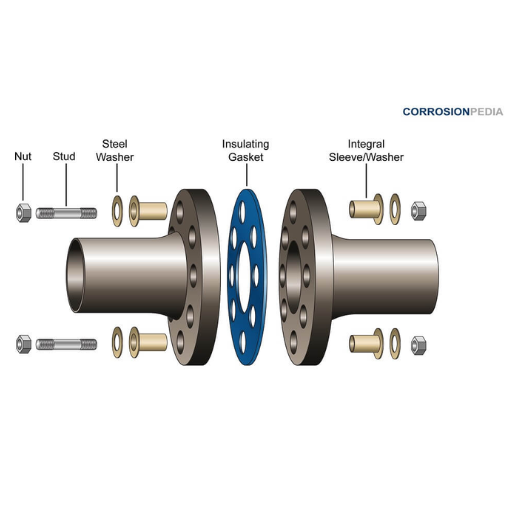
1. Material Selection and Compatibility
Galvanic Series Consideration
Select metals that are close to each other in the galvanic series to minimize potential differences. When dissimilar metals must be used, ensure proper isolation and protection measures.
Material Selection Best Practices:
- Choose metals with similar electrochemical potentials
- Use the same metal throughout the system when possible
- Avoid large cathode-to-anode surface area ratios
- Consider the specific environment and exposure conditions
- Consult galvanic compatibility charts during design
2. Protective Coatings and Surface Treatments
| Coating Type | Application | Durability | Cost | Best Use Cases |
|---|---|---|---|---|
| Hot-Dip Galvanizing | Steel immersion in molten zinc | 25-50 years | Medium | Structural steel, outdoor applications |
| Electroplating | Electrochemical deposition | 5-15 years | Low-Medium | Small parts, decorative items |
| Spray Galvanizing | Zinc spray application | 15-25 years | Medium | Field repairs, large structures |
| Anodizing | Aluminum oxide formation | 20-30 years | Medium-High | Aluminum components |
| Polymer Coatings | Paint systems, powder coating | 5-20 years | Low-Medium | General protection, aesthetics |
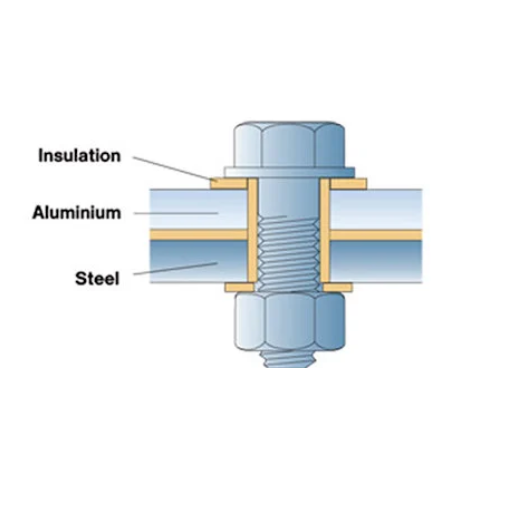
3. Electrical Isolation Techniques
🔧 Isolation Methods
- Insulating gaskets – Rubber, plastic, or composite materials
- Non-conductive fasteners – Nylon, ceramic, or polymer bolts
- Dielectric coatings – Electrically insulating barrier layers
- Physical separation – Air gaps or non-conductive spacers
- Insulating sleeves – For bolts and fasteners
4. Sacrificial Anode Systems
Sacrificial anodes are highly effective for protecting larger structures, particularly in marine and underground applications.
| Anode Material | Environment | Lifespan | Applications |
|---|---|---|---|
| Zinc | Seawater, soil | 2-20 years | Ship hulls, pipelines |
| Magnesium | Freshwater, soil | 5-15 years | Underground tanks, pipes |
| Aluminum | Seawater | 5-25 years | Offshore structures |
5. Environmental Control Measures
1Moisture Control: Use dehumidifiers, proper ventilation, and moisture barriers
2Drainage Design: Prevent water accumulation at metal junctions
3Sealants: Apply weatherproof sealants to prevent electrolyte access
4Ventilation: Ensure adequate air circulation to reduce humidity
5Regular Cleaning: Remove salt deposits and contaminants
Design Strategies for Prevention
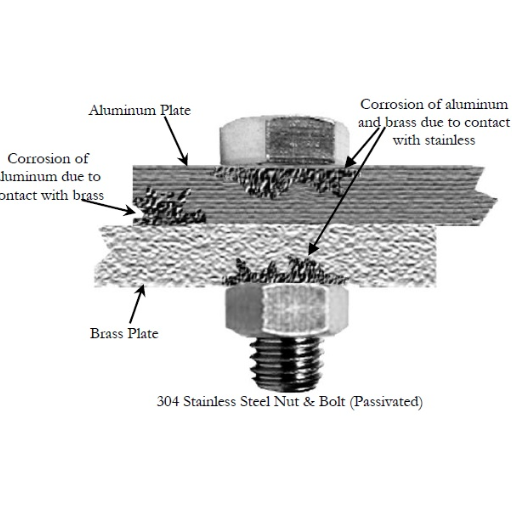
Engineering Approaches
- Minimize contact area: Reduce the surface area where dissimilar metals touch
- Avoid crevices: Design joints to prevent water and debris accumulation
- Use compatible fasteners: Select bolts and screws made from compatible materials
- Plan for maintenance: Design accessible inspection and replacement points
- Consider galvanic series: Position more noble metals downstream in fluid systems
Maintenance and Inspection Guidelines
Regular Inspection Schedule
| Environment | Inspection Frequency | Key Indicators | Action Items |
|---|---|---|---|
| Marine/Coastal | Every 3-6 months | Pitting, discoloration, coating damage | Clean, recoat, replace anodes |
| Industrial | Every 6-12 months | Surface roughness, metal thinning | Monitor coating integrity, document changes |
| Atmospheric | Annually | Rust stains, coating failure | Touch-up coatings, check fasteners |
| Underground | Every 2-3 years | Anode consumption, soil conditions | Replace anodes, test electrical continuity |
References
-
Classic Galvanic Corrosion – NOAA – Highlights the use of sacrificial anodes to prevent galvanic corrosion in marine environments.
-
Galvanic Corrosion on Aluminum – University Corporation for Atmospheric Research (UCAR) – Discusses the galvanic series, material selection, and design considerations to minimize galvanic corrosion.
Frequently Asked Questions (FAQ)
What is galvanic corrosion and how does it occur?
Galvanic corrosion, with its more precise name bimetallic corrosion, is the process of one metal corroding preferentially from its are of contact with another metal. Usually, this takes place when two different metals come in touch with each other and an electric current forms through them while in touch. The metal which produces the current is the anode, and so it requires further protection as the other retained metal is the cathode. The bimetal situation must be eliminated or measures are taken to maintain it, else both metals will be subjected to corrosive effects.
Can galvanic corrosion be prevented in metallic structures?
The way to deal with the galvanic corrosion is to decrease electric contact between the metals. The electric link can be reduced by using isolating fasteners, coatings that do not conduct, or even compatible materials. In highly corrosive environment, the first and simple way is to use materials that are not highly reactive with other materials. In the isolated fasteners the corrosion is the least, and in the coatings it is faster, and with the greases the newest and the approach the easiest. All of them enhance the time the metal can be used.
What is the role of galvanization in the prevention of corrosive action?
Galvanization is a delayed chemical reaction, which is why it is acknowledged as superior. The application of the layer of zinc to the steel or iron provides long-lasting protection to the metal. It is by the formation of the sacrificial anode of zinc which corrodes in place of the underlying metal. For the protection of steel against corrosion, this move is particularly essential. It is in offshore applications that are exposed to saline waters that this move helps a lot.
How does utilising a ‘sacrificial’ anode aid in preventing corrosion?
The intended insertion of a sacrificial anode, usually zinc or magnesium, conductively connected to the noble metal, protects steel from galvanic corrosion. In the process, the sacrificial anode is slowly degraded in the place of the steel, ensuring no corrosion in the system. This method can be noted to be widely effective in applications such as boat hulls, as well as underwater pipelines treatment due to the water immersion.
What are the electronic criteria needed to cause galvanic corrosion?
The purpose of galvanic corrosion is to allow two different metals to come into electrical contact through an electrolyte which in most cases is salt water. Once in contact, the electrical will corrode the metal which has the highest electric potential. In order to prevent this from happening, the metals must not be connected in any to prevent the electrical flow of the current.
Can galvanic corrosion be prevented with corrosion inhibitors?
Absolutely. Corrosion inhibitors are effective in combatting galvanic corrosion. Employing them can slow the corrosion of the metals that function as anodes, while enhancing those of the cathodes. In the process, a protective barrier is established that targets the metal surface, lowering the level of electric activity which ordinarily leads to corrosion.
How does the difference between the electrode potential affect galvanic corrosion?
The implication is that corrosion is most prevalent when there is a distinct gap in the order of electrode potential between two metals. When the anodic metal has an extremely low electrode potential as opposed to that of the other, it tends to corrode significantly faster. The application of this principle can therefore be used in the context of protecting metals that are at a high risk of corrosion.
What materials should not be used to prevent galvanic corrosion?
Part of the process of preventing galvanic corrosion involves the use of materials that are quite the opposite of each other with respect to their electrode potential, since they are going to be in contact with each other. For example, alloys of aluminum and copper should not be used because they tend to corrode faster than other materials. When it comes to protecting the metals from any kind of corrosion, one may choose to use materials that are part of the metallic series or materials that do not interact with each other and need to be protected using non-conductive coatings.
What are the most common sign of galvanic corrosion?
In the case of galvanic corrosion, common indicators are the pitting, discolouration, and surface roughness on the anodic metal. Furthermore, you will also find patterns where the metal is breaking down or has the surface peeling up, which makes it irregular. To prevent additional potential damage, catching the above signs as soon as possible and undergoing proper inspection and maintenance is extremely crucial.