Industries rely on metals for their high rate of heat conduction, which facilitates numerous applications that depend on efficient heat transfer. Among these metals, stainless steel occupies a special position due to its vitality, strength, corrosion resistance, and unique thermal conductivity properties. This comprehensive guide explores the thermal conductivity of stainless steel and compares it with other metals, providing engineers and materials scientists with essential information for material selection and design.
Introduction to Thermal Conductivity
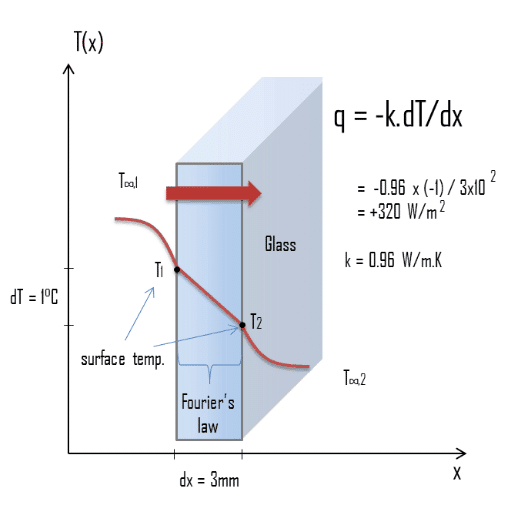
Heat transfer occurs when there is a temperature difference between two points. Thermal conductivity measures a material’s ability to conduct heat—materials with high thermal conductivity transfer heat efficiently, while those with low conductivity act as insulators.
Key Point: The thermal conductivity of stainless steel is lower compared to other metals like copper and aluminum, making it more suitable for heat-resistant applications and thermal insulation.
Definition of Thermal Conductivity
Thermal conductivity refers to the capacity of a material to transfer heat through a given thickness under a temperature gradient over time. It is measured in W/m·K (Watts per meter-Kelvin).
- High conductivity materials (e.g., copper, aluminum) efficiently dissipate heat
- Low conductivity materials (e.g., ceramics, plastics) work as effective insulators
- Conductivity depends on lattice vibrations, free electron motion, and nanoscale features
Importance in Material Selection
Selecting appropriate materials is critical in engineering and design, affecting:
- Functionality and durability
- Energy consumption and efficiency
- Device lifespan and performance
- Cost-effectiveness of operations
Modern materials like aerogels, phase change materials, and advanced polymers offer improved thermal properties for specialized applications including renewable energy systems and microelectronics.
Applications Across Various Industries
Electronics and Computing
Thermal management is crucial in electronics to prevent overheating of components like CPUs, GPUs, and microchips. High thermal conductivity materials are essential:
- Copper (~385 W/m·K) – Used in heat sinks and thermal spreaders
- Aluminum (~205 W/m·K) – Lightweight alternative for thermal management
- Advanced materials – Graphene-based components and phase change materials (PCMs)
Aerospace and Aviation
Materials in aerospace must withstand extreme thermal conditions:
- Ceramic Matrix Composites (CMCs): 8–30 W/m·K for engine components
- Silica tiles: ~0.057 W/m·K for spacecraft thermal protection
- High-temperature resistance for atmospheric reentry
- Low-temperature stability for space applications
Construction and Civil Engineering
Building materials are selected to maintain thermal comfort and energy efficiency:
| Material | Thermal Conductivity (W/m·K) | Application |
|---|---|---|
| Concrete | ~1.8 | Structural elements |
| Brick | ~0.69 | Walls and facades |
| Polyurethane foam | ~0.03 | Insulation |
| Mineral wool | 0.04 – 0.045 | Thermal insulation |
Automotive Industry
Automotive materials must endure large temperature variations in engines, exhaust systems, and electric vehicle batteries:
- Aluminum alloys and cast iron for engine blocks
- Thermal gels and heat pipes for EV battery cooling
- Heat-resistant materials for exhaust systems
Renewable Energy Systems
Solar and geothermal systems require materials with specific thermal properties:
- Solar panels: Aluminum frames and temperature-resistant glass (>1.05 W/m·K)
- Geothermal systems: High conductivity metals in heat extraction pipes
Thermal Conductivity of Different Metals
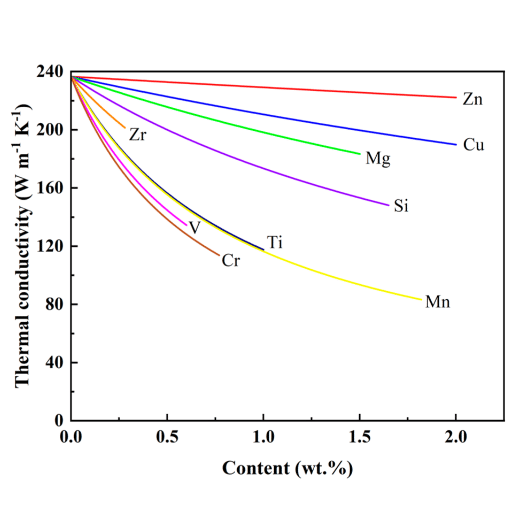
Metal thermal conductivity varies significantly based on composition, purity, and atomic structure. Free electrons in metals facilitate rapid heat transfer, with some metals conducting heat far more efficiently than others.
Overview of Thermal Conductivities
| Metal | Thermal Conductivity (W/m·K) | Key Applications |
|---|---|---|
| Silver | ~430 | Specialized electronics (expensive) |
| Copper | ~400 | Electrical conductors, heat sinks |
| Gold | ~317 | High-end electronics |
| Aluminum | ~237 | Heat sinks, lightweight applications |
| Carbon Steel | 45-60 | Heat exchangers, structural components |
| Stainless Steel | 15-25 | Corrosion-resistant applications |
| Titanium | ~21.9 | Aerospace, medical implants |
Comparative Analysis
Understanding thermal conductivity differences helps engineers select appropriate materials:
- High conductivity metals (copper, silver): Ideal for cooling systems and heat exchangers
- Moderate conductivity metals (aluminum): Balance between performance and weight
- Low conductivity metals (stainless steel): Preferred when heat retention or insulation is needed alongside corrosion resistance
Innovation: Aerogels represent cutting-edge insulation materials with thermal conductivity as low as 0.015 W/m·K, opening new possibilities in construction and aerospace applications.
Factors Affecting Thermal Conductivity
Atomic Structure and Bonding
- Metals: Free electrons provide pathways for efficient energy transfer
- Ceramics and polymers: Rely on phonon propagation, resulting in lower conductivity
- Atomic packing density influences heat transfer efficiency
Temperature Effects
Temperature significantly impacts thermal conductivity:
- Metals: Generally experience decreased conductivity at higher temperatures due to electron scattering
- Insulators: Often show increased conductivity with temperature due to enhanced phonon contribution
Microstructure
Material microstructure affects thermal performance:
- Impurities disrupt energy flow
- Voids and grain boundaries scatter heat carriers
- Nanostructured features can optimize thermal characteristics
- Additive manufacturing enables controlled microstructures
Stainless Steel and Its Thermal Conductivity
Stainless steel is renowned for excellent mechanical properties, corrosion resistance, and longevity. However, its thermal conductivity is notably lower than other common metals.
Understanding Stainless Steel Thermal Conductivity
Typical stainless steel grades (304 and 316) exhibit thermal conductivity of approximately 14-16 W/m·K at ambient temperature—significantly lower than copper’s ~400 W/m·K. This lower conductivity results from:
- Alloy composition (chromium, nickel, manganese)
- Disrupted lattice vibrations
- Complex microstructure
Important Note: Stainless steel’s thermal conductivity decreases with increasing temperature, a critical consideration for high-temperature applications in aerospace and power generation.
Grades of Stainless Steel and Their Conductivities
Stainless steel comes in four main categories, each with distinct thermal properties:
| Type | Common Grades | Thermal Conductivity (W/m·K) | Key Characteristics |
|---|---|---|---|
| Austenitic | 304, 316, 316L | 14-16 | Excellent corrosion resistance, non-magnetic, widely used |
| Ferritic | 430 | 23-27 | Better thermal performance, magnetic, moderate corrosion resistance |
| Martensitic | 410, 420 | ~25 | High strength and hardness, less corrosion resistant |
| Duplex | Various | Intermediate | Balance of austenitic and ferritic properties |
Applications of Stainless Steel as a Thermal Conductor
Heat Exchangers
Stainless steel heat exchangers are extensively used in chemical processing, power generation, and HVAC systems. Grade 316L is particularly valued for chloride corrosion resistance while maintaining adequate thermal performance.
Cooking and Kitchenware
Stainless steel cookware provides uniform heat distribution. While its thermal conductivity is lower than aluminum or copper, cladding processes create durable, warp-resistant cookware.
Industrial Furnaces
High-temperature grades (310, 253MA) withstand temperatures beyond 1800°F (982°C), making them ideal for metallurgy and industrial heating applications.
Cryogenic Equipment
Grades 304 and 316 maintain stability and efficiency at cryogenic temperatures, suitable for tanks and pipelines in low-temperature applications.
Automotive Exhaust Systems
Stainless steel’s relatively low thermal expansion coefficient and adequate thermal conductivity make it suitable for exhaust systems that experience significant temperature variations.
Comparing Carbon Steel and Stainless Steel
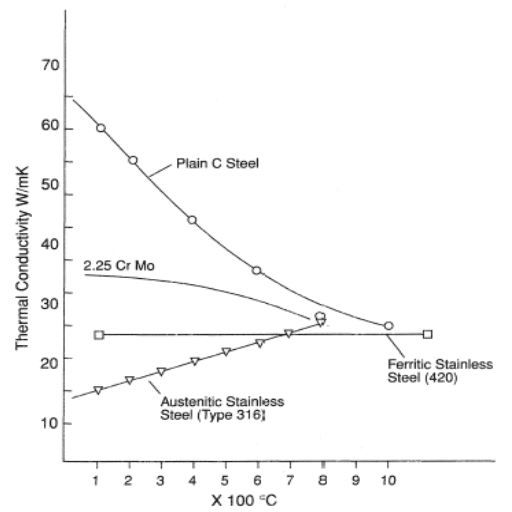
Fundamental Differences
Carbon Steel:
- Primarily iron and carbon (0.3% – 2.0% carbon content)
- May include manganese, silicon, phosphorus, sulfur
- Susceptible to corrosion and rust
- Higher thermal conductivity: 45-60 W/m·K
- Greater strength and rigidity
Stainless Steel:
- Minimum 10.5% chromium content
- Forms protective oxide layer
- Excellent corrosion and oxidation resistance
- Lower thermal conductivity: 15-20 W/m·K
- Glossy, attractive surface finish
- Enhanced with nickel, molybdenum, nitrogen
Thermal Conductivity Comparison
The thermal conductivity difference between carbon steel and stainless steel stems from their composition:
- Carbon steel: Simpler structure allows better heat conduction
- Stainless steel: Chromium and nickel stabilize austenite structure, impeding heat flow
- Carbon content affects conductivity—lower carbon content generally means higher conductivity
Applications and Implications
Heat Exchangers and Boilers
Carbon steel is preferred for applications requiring rapid heat transfer and efficient thermal performance, helping reduce energy costs.
Pipeline Systems
Carbon steel pipelines minimize temperature losses during fluid transportation, crucial in oil and gas industries.
Industrial Furnaces
Carbon steel furnace linings eliminate temperature differentials, enhancing efficiency in smelting and heat treatment processes.
Automotive Components
Both steels are used, with carbon steel in exhaust and engine parts for effective heat dissipation, and stainless steel where corrosion resistance is essential.
Power Generation
Carbon steel is utilized in solar heating systems for efficient heat transfer and storage without temperature-induced deformation.
Poor Thermal Conductors: Understanding Limitations
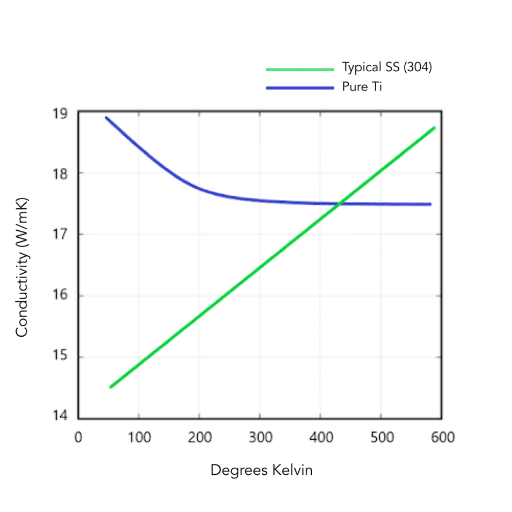
Identifying Poor Conductors
Materials like wood, rubber, and certain plastics are poor thermal conductors, making them valuable for insulation applications. Among metals, some exhibit relatively low thermal conductivity:
| Material | Thermal Conductivity (W/m·K) | Common Applications |
|---|---|---|
| Lead | ~35 | Radiation shielding |
| Titanium | ~21.9 | Aerospace, medical |
| Stainless Steel | 14-25 | Pot handles, structural insulators |
Impact on Performance
Insufficient thermal conductivity can lead to:
- Localized heating and hot spots
- Reduced system efficiency
- Shortened component lifespan
- Performance degradation in electronics and computing
Advanced Alternatives
Modern materials science offers innovative solutions:
- Graphene and carbon nanotubes (CNTs): Outstanding thermal conductivity for composite applications
- Phase Change Materials (PCMs): Absorb latent heat to manage temperature surges
- Boron nitride (BN) ceramics: Excellent thermal and dielectric properties for electronics
- Additive manufacturing: Enables customized thermal management solutions
References
-
Thermal Conductivity – HyperPhysics (Georgia State University)
A comprehensive table of thermal conductivity values for various materials, including steel.
Visit the site -
Is Stainless Steel a Good Conductor of Heat? – Butler University
An exploration of the thermal conductivity of stainless steel compared to other metals, with detailed analysis.
Visit the site -
High-Temperature Characteristics of Stainless Steels – Stanford University
A document discussing the physical properties of stainless steels, including thermal conductivity and linear expansion.
Visit the site
Frequently Asked Questions (FAQ)
What is the thermal conduction of steel?
Steel thermal conductivity changes with grades of the steel though it is usually between 15 and 25 W/mK. That property is particularly important where heat needs to be transferred, for example in the food processing or electronic cooling arrangements.
How do the thermal conductivities of various metals differ?
Looking at the thermal conductivities of metals leads one to note that aluminum having a thermal conductivity of about 205 W/mK is a very good conductor of heat. On the other hand steel showing a lower thermal conductivity is significant in many places where heat needs to be retrieved.
What changes the thermal conductivities of metals?
Thermal conductivity is a physics concept which tries to explain transport of heat and particularly the reasons of heat transfer in different materials. A number of physical conditions such as changing temperature, addition of substances and transformation of a material medium can affect scientific values of thermal conductivity of metals. Carbon steel, more or less, effectiveness in conducting heat varies depending on alloy components and post production heat engineering techniques such as annealing.
What grades of stainless steel possess maximum heat conductivity?
There are grades, among stainless steel such as 316 and 304 that can be said possess relatively high thermal conductivity unlike the rest of the stainless steels. But the ability of these metals to undergo heat exchange still falls below the level of copper and aluminum.
How much specific heat does stainless steel have?
The specific heat capacity of the stainless steel material stands at about 500 J/Kg/K, translating to the amount of heat that one unit of mass of the material has to be exposed to, to raise its temperature by one degree kelvin. When one looks at this characteristic and its steel thermal conductivity, one will see why stainless steel is so versatile and important for thermal application purposes.
What is the effect of temperature on the thermal expansion of metals?
As the temperature of a given metallic object is increased, it expands and contracts when cooled. This change can be sometimes useful, for example, in altering heat flow in situ such as shielding heat in food processing equipment, but cares should always be paid to the consequences of increased thermal conductivity changes in a metal.
Why’s the significant addition of carbon nanotubes to the thermal conductivity case?
Carbon nanotubes, with their extraordinary structures, are known to display thermal conductivities which are often higher than those of metals. Because structural features of these materials enable them to be outstanding for heat dissipation purposes, these materials are applied in areas where higher thermal action is needed as in composite and electronic devices.
In what way is thermal conductivity determined?
Thermal conductivity is described in terms of the number of watts crossing unit thickness or area of the material across a given temperature gradient, and the unit of measurement is W/m°K. The thermal management knowledge of the properties of material objects such as steel and copper also ensures that practitioners in related fields do so comprehensively.







