Passivating stainless steel is one of the processes that can be used to reduce the chance of premature failure of the material. Stainless steel is widely used due to its resistance to corrosion in industrial, medical or home equipment, however, this resistance can be achieved and sustained over time only if the treatment is properly done. This article explores the principles and the practice of passivation and explains the need to protect stainless steels from rust or deterioration.
What is Passivation?
The process of passivation involves the strengthening of the stainless steel against corrosion through a chemical procedure. This is achieved by the mechanical removal of all free iron and other impurity ions present on the surface of the metal followed by the formation of a thin oxide layer. The oxide layer serves the purpose of preventing any rust or attack on the steel.
Definition of Passivation
Passivation is a physical and chemical process of creating a thin oxidized metal layer on the surface of stainless steel and protecting it against atmospheric corrosion. This is achieved by washing a metal surface clean of all foreign materials and passivating it with acids such as nitric or citric to form inert coatings which don’t get attacked by the surrounding environment.
Importance of Passivating Stainless Steel
- Enhanced Durability: Increases resistance to corrosion and extends material lifespan
- Industry Applications: Critical for sensitive industries like aerospace, medical, and food processing
- Environmental Benefits: Growing trend toward eco-friendly and non-harmful passivation methods
- Cost-Effectiveness: Reduces maintenance costs and prevents premature component failure
Common Methods of Passivation
| Method | Acid Type | Environmental Impact | Advantages |
|---|---|---|---|
| Nitric Acid Passivation | Strong acids | Higher environmental impact | Traditional method, well-established process |
| Citric Acid Passivation | Biodegradable solutions | Environmentally friendly | Less toxic, sustainable, aligns with green initiatives |
The Passivation Process
Steps in the Passivation Process
- Cleaning the Surface: Remove all contaminants including oils, grease, dirt, or residues using detergents or specialized cleaning agents
- Pickling (if needed): Address severe surface imperfections and scaling using acid solutions
- Passivation Treatment: Apply passivating acid solution (nitric or citric acid) to remove free iron and promote protective oxide layer formation
- Thorough Rinsing: Rinse with deionized water to remove all remaining chemicals
- Drying and Inspection: Complete drying followed by testing using water break tests or advanced analytical methods
Using Citric Acid for Passivation
Citric acid has become the preferred stainless steel passivation agent due to its environmentally beneficial and efficient characteristics. Unlike traditional nitric acid, citric acid is:
- Completely biodegradable and environmentally respectful
- Less harmful to workers and the environment
- Capable of providing corrosion resistance equal to or better than conventional treatments
- Compatible with newer stainless steel alloys used in medical, aerospace, and food processing
- Cost-effective with reduced health hazards and disposal costs
Benefits of Passivating Stainless Steel

Enhanced Corrosion Resistance
Creates a protective oxide layer that resists rusting and chemical attacks, even in hostile environments like marine or industrial settings.
Improved Aesthetic Appearance
Removes surface impurities and ensures a uniform oxide layer for a cleaner, brighter, and more polished appearance.
Extended Component Life
Significantly increases the lifespan of stainless steel components, reducing maintenance and replacement costs.
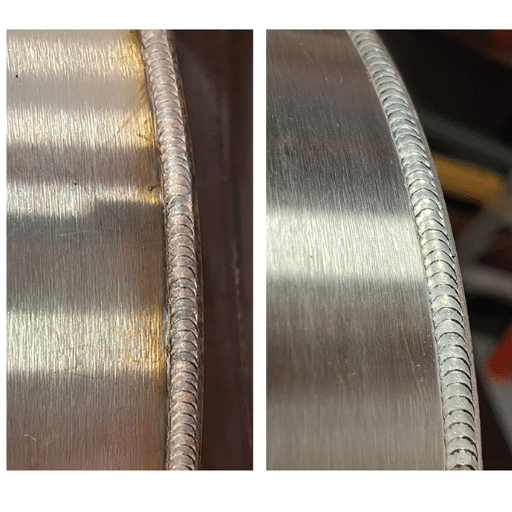
Passivation of Stainless Steel Welds

Passivation treatment in stainless steel welds is crucial for improving corrosion resistance by removing contaminants such as free iron that may have been deposited during welding. The welding process disrupts the protective chromium oxide layer due to intense heat, making these areas susceptible to corrosive attack.
Challenges in Passivating Welded Areas
- Heat tint or oxide scale coverage that requires removal
- Disrupted protective chromium oxide layer
- Need for thorough cleaning prior to passivation
- Potential for localized corrosion if improperly treated
Techniques for Effective Weld Passivation
- Thorough Cleaning: Remove contaminants including grease, oils, and oxide scale through mechanical means
- Chemical Treatment: Apply nitric or citric acid solutions to form protective oxide layer
- Advanced Technologies: Utilize electrochemical cleaning with electrical currents for enhanced effectiveness
- Quality Testing: Conduct iron contamination or salt spray tests to ensure effectiveness
Testing and Validation

| Test Method | Purpose | Standard |
|---|---|---|
| Ferroxyl Test | Detects iron contamination on surface | ASTM A967 |
| Salt Spray Test | Judges corrosion resistance under aggressive conditions | ASTM B912 |
| Copper Sulfate Test | Verifies passive oxide layer presence | ASTM A380 |
Rust Prevention and Maintenance
Rust prevention and maintenance are fundamental pillars for the longevity and reliability of metalwork across different sectors. Modern approaches include:
- Protective Coatings: Paint or specialized rust inhibitors
- Advanced Technology: Vapor corrosion inhibitors (VCIs) and nanotechnology-based sealants
- Environmental Controls: Managing humidity and avoiding prolonged chemical exposure
- Material Selection: Using corrosion-resistant alloys for harsh conditions
References
-
Citric Acid Passivation Study – NASA – Explains how passivation solutions work and their effects on stainless steel surfaces.
-
Role of Structure and Oxidation States in the Passivation of Stainless Steel – Louisiana Tech University – Studies the early stages of oxidation in stainless steel using advanced theoretical calculations.
Frequently Asked Questions (FAQ)
What is the passivation of stainless steel parts meant for?
It is passivation of the stainless steel parts so as to increase the corrosion resistance of these items. The process is to lay down a protective layer on the surface of the steel to act against rusting and other forms of degradation occasioned by environment factors like oxygen exposure or chloride presence.
How do stainless steel chemical passivation treatments work?
Chemical passivation treatments for stainless steel consist in allowing the parts to be immersed in a nitric acid solution or an acid bath which tends to remove iron particles and impurities. The process fosters the formation of a thin, protective oxide layer on the surface as a barrier that considerably enhances corrosion resistance of the stainless steel.
What are the impacts of passivation on the corrosion resistance of stainless steel?
The impact of passivation on corrosion resistance of stainless steel is quite noted. For, the passivation treatment of stainless steels produces a reduction of corrosion, especially in an environment where chloride ions are present, thus ensuring a greater life span to the stainless steel parts and components.
Can stainless steel welds be passivated?
Yes, stainless steel welds can be passivated. Stainless steel welds improve corrosion resistance after passivation in the areas that have been welded. Cleaning and passivating welds on stainless steel is important for removing contaminants that could cause corrosion.
What grade of stainless steel responds best to passivation?
Higher-grade stainless steel that responds to passivation would normally include austenitic grades such as 304 and 316, which offer excellent corrosion resistance. Even though other grades go through passivation successfully, their corrosion properties remain enhanced in depleting environments.
What is the passivation protocol with the nitric acid bath?
When using the nitric acid bath, the stainless steel parts are first cleaned, and this is followed by immersion in a diluted nitric acid solution. The solution dissolves iron particles and helps in the formation of a passive oxide layer, thus enhancing corrosion resistance on the surface of the stainless steel.
How does one clean and passivate stainless steel samples?
Effective methods of cleaning and passivating stainless steel begin with cleaning to remove surface contaminants such as dirt, grease, and others. Cleaning can be performed using sodium hydroxide or phosphoric acid solutions. The samples are then passivated by treating them with nitric acid solution to accept the passive state on the surface.
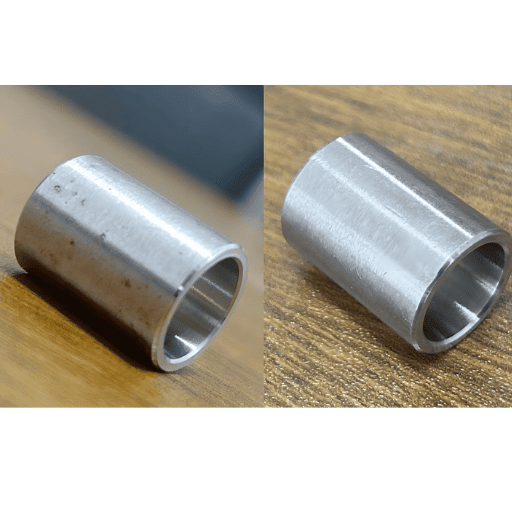
What are the advantages of passivating stainless steel parts after machining?
The objectives of performing the passivation of stainless steel parts after machining include removing any iron particles that may have been introduced during machining, as well as improving corrosion resistance. This is essential for the protection of stainless steel parts in applications where severe stress is involved.
Is a passivation test necessary for stainless steel?
It is not really necessary to put stainless steel to a passivation test, but such a test is suggested to ascertain whether the passivation was effective or not. The purpose of the test is to ensure that the protective layer was actually formed and that the stainless steel will be able to withstand adversaries in time due to actual corrosion.







