Stainless steel is famous for its long durability, strength and more so its ability to remain rust and corrosion free. However, not every stainless sheet is the same. Depending on the application and how often either tarnishing or corrosion becomes a problem in stainless steel. In order to select the best kind of stainless steel, one must learn why and how stainless steel resists rusting. This article examines the science of stainless steel corrosion resistance in greater depth. It also evaluates the different types of stainless steel that are available, as well as their distinguishing features.
Introduction to Stainless Steel
The combination of iron, chromium, and other chemical elements like nickel and molybdenum produce a very tough material that holds many practical uses: stainless steel. This type of steel is unique as unlike other steels it does not seem to corrode, or rust; this is because it contains chromium levels of not less than 10.5%. It forms a protective layer on the surfaces which protects them from further oxidation.
Definition and Composition
A combination of metals are used to create stainless steel; this includes iron, chromium, nickel in majority and sometimes minute proportions of elements like:
- Carbon
- Molybdenum
- Manganese
Historical Development of Stainless Steel
Stainless steel’s origins can be traced back to the twentieth century. The first stainless steel was developed by British metallurgist Harry Brearley who started production in 1913. Testing several elemental combinations, chiefly including more than 10.5% chromium, he created an alloy that resists oxidation and corrosion.
Importance in Modern Manufacturing
Stainless steel’s usage is common in industrial manufacturing today due to its:
- Strength and durability
- Corrosion resistance
- Multipurpose applications
- Role in renewable energy (windmills and solar panels)
- Use in 3D printing technologies
Understanding Corrosion Resistance

What is Corrosion?
Corrosion is an unavoidable process where metals are damaged due to a chemical or electrochemical interaction with their surroundings. There are different types of corrosion:
- Uniform corrosion – Even deterioration across the surface
- Pitting corrosion – Localized holes or pits
- Crevice corrosion – Occurs in confined spaces
- Galvanic corrosion – Between dissimilar metals
How Stainless Steel Resists Corrosion
Stainless steel corrosion resistance is primarily due to chromium content (minimum 10.5%). When chromium comes into contact with oxygen, a thin layer of stable chromium oxide forms on the surface, creating a protective barrier that:
- Prevents oxygen and water vapor from reaching the steel
- Self-heals when scratched (if sufficient oxygen is available)
- Provides long-term protection against rust formation
Factors Affecting Corrosion Resistance
| Factor | Impact on Corrosion Resistance |
|---|---|
| Chromium Content | Higher chromium content improves passive layer formation |
| Molybdenum | Reduces pitting and crevice corrosion in chloride environments |
| Nickel | Enhances overall corrosion resistance and ductility |
| Environmental Conditions | Temperature, humidity, and chemical exposure affect performance |
| Surface Finish | Smoother surfaces provide better corrosion resistance |
| Maintenance | Regular cleaning prevents passive film breakdown |
Types of Stainless Steel
Austenitic Stainless Steel
Characteristics:
- Most common type of stainless steel
- Excellent corrosion resistance
- Non-magnetic nature
- High ductility
- High chromium (>16%) and nickel content
Common Grades: 304, 316
Applications: Food industry, architecture, surgical components
Ferritic Stainless Steel
Characteristics:
- Mainly iron and chromium (10.5-27%)
- Very little or no nickel
- Magnetic properties
- Good resistance to stress corrosion cracking
- More cost-effective than austenitic grades
Common Grades: 430, 409
Applications: Automotive industry, household equipment
Martensitic Stainless Steel

Characteristics:
- High strength and hardness
- Modest chromium content
- Often contains carbon
- Less corrosion resistant than other types
- Can be heat treated
Common Grades: 410, 420
Applications: Knives, tools, turbines
Duplex Stainless Steel
Characteristics:
- Combination of austenite and ferrite structures
- High strength
- Superior corrosion resistance
- Resistant to chloride-induced stress corrosion cracking
Common Grade: 2205
Applications: Chemical and marine industries
Precipitation-Hardening Stainless Steel
Characteristics:
- Very high strength and hardness
- Requires specialized heat treatment
- Excellent mechanical properties
Common Grade: 17-4 PH
Applications: Aerospace, nuclear applications, medical devices
Performance in Various Environments
Marine Environments
Marine environments present tough conditions with high salinity, pressure, and moisture. Grade 316 stainless steel is highly preferred due to its molybdenum content, which provides excellent resistance to:
- Pitting corrosion
- Crevice corrosion
- Salt water exposure
Marine Applications:
- Ship Construction: Hull protection against corrosive salt effects
- Offshore Rigs: Essential for oil and gas production equipment
- Underwater Pipelines: Oil and gas transportation systems
- Seawater Desalination Plants: Processing equipment and frameworks
Food and Beverage Industry
Stainless steel importance in food and beverage industry is attributed to:
- Sanitary conditions maintenance
- Non-porous surface that prevents bacterial growth
- Compliance with health and safety regulations
- Easy cleaning and maintenance
Chemical Processing Plants
Used in manufacturing of:
- Reactors
- Pipelines
- Storage tanks
- Processing equipment for aggressive chemicals
Rust Prevention Techniques

Protective Coatings
Advanced protective coatings extend equipment life and include:
- Epoxy coatings: High adhesion and chemical resistance
- Polyurethane coatings: Excellent durability
- Ceramic-based coatings: Superior temperature resistance
Regular Maintenance Practices
Essential maintenance procedures include:
- Routine inspections – Early detection of issues
- Surface cleaning – Prevention of contamination buildup
- Following manufacturer guidelines – Proper care procedures
- Professional maintenance – Periodic expert assessment
- Avoiding abrasive cleaners – Protecting surface integrity
Choosing the Right Alloy for Durability
Selection criteria should consider:
- Environmental conditions
- Mechanical stress requirements
- Application type
- Temperature ranges
- Budget constraints
References
-
A Faster, Cheaper Way to Restore Stainless Steel’s Corrosion Resistance – University of Wisconsin-Madison – Explains the role of chromium in stainless steel’s corrosion resistance and methods to restore it.
-
High-Temperature Characteristics of Stainless Steels – Stanford University – Discusses the selection of stainless steels based on corrosion resistance and mechanical properties.
-
Corrosion of Stainless Steels – Harvard ADS – Provides an overview of stainless steels as corrosion-resistant iron-base alloys.
Frequently Asked Questions (FAQ)
What is the corrosion resistance of stainless steel?
The chemical element chromium allows good corrosion resistance of stainless steels due to the chromium oxide present in its composition and its ability to polish itself. As the oxide layer is free standing on the surface of the metal, there won’t be a room for rust, consequently a very good option where corrosion is the risk.
What is the difference between stainless steel and carbon steel when it comes to their corrosion resistance?
Carbon steel to a very degree is almost non existent to any oxidizing condition like humidity or air and the substances presented in them. On the other hand, with the aid of chromium and alloying elements containing other corrosion resistant element such as nickel, the rust problem associated with carbon steels is not an issue with stainless steels.
In terms of corrosion resistance, what are the kinds of stainless steel?
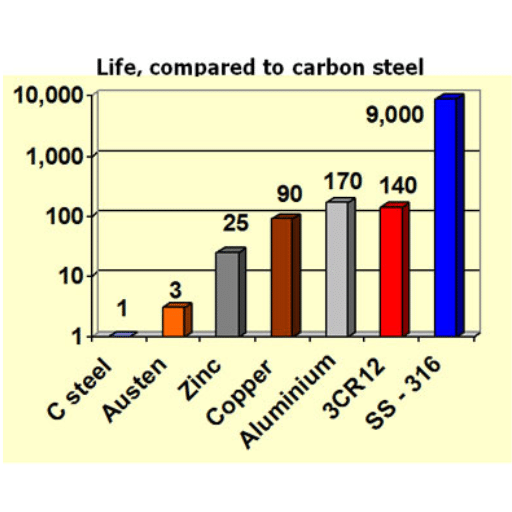
Different types of stainless steel exhibit different corrosion resistance characteristics. For instance, for instance, we have type 304 stainless steel, which enjoys excellent resistance to normal corrosion conditions, and then we have type 316 stainless steel, which is a better grade of steel and much more resistant to corrosion due to the presence of molybdenum. As a result, 316 is very suitable for use in sea environments and in every medium that is exposed to salt water.
What is the mechanism of stainless steel corrosion and what are the different types of stainless steel corrosion?
There are certain forms of corrosion that are prevalent on stainless steel, and they include pitting, intergranular and galvanic. Pitting is usually the most encountered localized corrosion, while intergranular usually occurs at the grain level. When two metals, one metal being less noble, are immersed in an electrolyte, galvanic corrosion will occur at the interface of stainless steel and that particular metal.
What about the content of steel, does it have an influence on resistibility to corrosion? In what way?
The level of steel purity, mainly the ratios of chromium and nickel, is the most important factor in relation to the corrosion resistance of the steel. Chromium, which is present at higher levels, promotes the development of a passivating oxide layer, whereas nickel contributes to the plasticity of the alloy, as well as rendering it oxidation and corrosion resistant or stain free.
Does stainless steel in specific circumstances develop rust?
Though, combating rust is the raison d’etre of stainless steel, it too can however corrode in certain environments, for example, if stored in highly acidic solutions like sulphuric acid or even within saline waters for prolonged durations. Proper care with appropriate cleaning practices such as using warm water and soap on the stainless steel will avoid the rusting occurrences within the metal over time.
For the purposes of stainless steel corrosion resistance, what is the importance of chromium in its composition?
Chromium is an essential alloy component in the composition of stainless steel and its inclusion in the alloy greatly improves stainless steel’s ability to resist corrosion. Upon exposure to air, it forms an extremely stable and thin coat of oxide on the surface of the metal that acts as a barrier to atmospheric elements that would otherwise cause the metal to rust, corrode, or sour.







