Electropolishing is an advanced metal finishing technique that further enhances stainless steel… A fact well known, stainless steel is strong, durable, and has endless uses, but its performance might be enhanced even further. Electropolishing improves corrosion resistance, smoothness, and cleanliness. Be it the Medical, Aerospace, Food, or Manufacturing industries, electropolishing needs to be incorporated to make the most of stainless steel components. This guide is tailored to reveal everything in one place, from the techniques and benefits of electropolishing to how it works and why it’s a revolutionary technique to accomplish the best results. Get ready!
What is Electropolishing and How Does it Work on Stainless Steel?

Electropolishing is a process that enhances the surface of stainless steel by material removal, more specifically, by applying a stronger polishing effect than usual. In this case, we immerse the stainless steel part into an electrolytic solution with some specific parameters of current and voltage. As a result, any surface bumps, roughness or dirt, etc. will be smoothed out and turned into a clean polished surface. With electropolishing, the corrosion resistance is further enhanced, cleanliness is improved, and the surface becomes aesthetically pleasing.
Understanding the Electrochemical Process Behind Electropolishing
Electropolishing works on the controlled anodic dissolution principle, which makes use of electrochemical reaction for surface material removal. When electricity is passed, the anode, which is the stainless steel workpiece, starts dissolving at an “electrochemical reaction” which is microscopic level in the electrolyte solution. This process works best on irregularly rough surfaces. High points (peaks) tend to dissolve quicker than low points (valleys), so these surfaces experience higher current densities. This micro-level trending achieves a smooth surface and helps in reducing cavities where dirt may settle.
Key Benefits and Data
- Corrosion Resistance Improvement:
Stainless steel’s resistant cap or passive oxide layer is enhanced by electropolishing which improves corrosion resistance. Studies have revealed that surfaces that have been electropolished showed up to 30% better performance in hostile environments compared to surfaces that were mechanically polished.
- Surface Roughness Reduction:
Electropolishing involves thorough cleansing of a workpiece in a least electrolyte bath. Such a practice is known to lower the roughness of a surface dramatically (Ra values). In the case of proprietary food and pharmaceuticals, industry standards for measuring cleanliness require surfaces to be smooth, which electropolishing achieves from a 50% to 75% reduction.
- Enhanced Cleanability:
Surfaces that were cleaned through this process are smoother on a microscopic level because there are fewer grooves to retain particles thus making such surfaces easier to clean.
- Visual Appeal:
For decorative and architectural purposes, polish aids in attaining a reflectance requirement that is 50% to 70% higher than the electropolished stainless steel.
This process is used in medicine, aerospace, and even semiconductors where performance matters. By applying electrochemistry with exact control, electropolishing including improving the functional and aesthetic value of the metal parts.
How Electropolishing Removes a Thin Layer of Metal
Electropolishing is accomplished via an electrolytic technique which selectively removes metal from a substrate surface by a given depth. To do this, the metallic part is submerged in an electrolytic solution, usually an acid blend, and electric direct current is passed. The metallic part—the workpiece—acts as an anode, while a cathodic stainless steel cylinder nearby acts as a cathode.
Electropolishing smooths out the surface features by dissolution of metal ions at the current emitting locations; this results in polishing of rough edges and removal of microscopic irregularities. The resultant surface is smooth and polished with even better corrosion resistance. Studies indicate that up to 30 microns of material removal is achievable by electropolishing, depending on current density, the composition of the electrolyte, time, and other variables of exposure. The just described process parameters would also require proper control of the temperature of the solution and stirring or movement of the electrolytic solution to maximize its effectiveness to achieve the desired results.
Electropolishing is favored because it enhances a metal’s surface topography as well as cleanliness by removing contaminants like oxides and other embedded particles. For precision-driven sectors such as medical devices, aerospace parts, and semiconductor fabrication, electropolishing achieves up to 50% roughness reduction, which helps meet stringent tolerances for improved operational and hygienic standards.
The Role of Temperature-Controlled Bath of Electrolyte
The temperature of the electrolyte bath is important in the electropolishing process, and it affects the quality and consistency of the results. Correct temperature settings are crucial in maintaining proper metallic surface dissolution period as well as preventing pitting and irregular etching. Studies suggest temperature for different metals and electrolytes ranges from 120°F to 200°F. Temperature control also enables uniform ion exchange, which is essential for smooth and polished surfaces. For stainless steel, a tightly controlled temperature range significantly improves corrosion resistance and increases reflective finish. Now, more advanced systems incorporate sensor-based tracking and auto correction systems for enhanced temperature control, which further optimizes process quality and reliability for electropolishing.
What Benefits Does Electropolishing Provide to Stainless Steel?
Electropolishing provides several key benefits to stainless steel, including:
- Improved Corrosion Resistance: With electropolishing, the protective oxide layer of the material is enhanced increasing its durability against rust and corrosion.
- Smoother Surface: By removing or polishing microscopic blemishes, uniform surfaces are achieved.
- Enhanced Aesthetics: Through polishing, a bright reflective finish is placed on the material, improving the overall appearance.
- Easier Cleaning: The clean surface allows for less contaminants to be trappe,d making cleaning and maintenance easier.
- Reduced Friction: Having smoother surfaces reduces friction significantly,y which can be crucial in machinery and medical devices.
Enhanced Corrosion Resistance of Stainless Steel
The surface imperfections and irregularities that can act as corrosion initiation points on stainless steel are efficiently removed through electropolishing, thereby significantly enhancing the metal’s corrosion resistance. This process also eliminates microscopic peaks and crevices, which help reduce the number of sites that corroding agents might accumulate in. Studies show that stainless steel that has undergone electropolishing shows increased corrosion resistance when placed in environments with high concentrations of chlorides, acids, and other aggressive chemicals. Research proves that when compared to untreated surfaces, the electropolished stainless steel is more capable of enduring a wider range of pH levels and retains its structural integrity for a longer duration during exposure to salt spray and humidity tests. This makes the metal especially useful in marine engineering, food processing, and pharmaceuticals, where strength in corrosive environments is highly valued.
How Electropolishing Improves Surface Finish
Electropolishing is tremendously effective in improving the surface finish of a metal by smoothing out the microscopic peaks and valleys. It raises the average roughness (Ra) value of the surface and can reduce the surface roughness by 50% relative to a polished finish. Surfaces with a lower Ra value are known to have better corrosion resistance, easier cleanability, and longer maintenance intervals, making electropolished surfaces perfect for sterilized and clean environments. The removal of surface contaminants and burrs results to a smoother surface which lowers friction and mechanical wear. These benefits have been proven in aerospace and medical device manufacturing, where precision and performance are highly demanded.
Benefits of Electropolishing Stainless Steel for Different Industries
Improved performance, lifespan, and functionality of stainless steel parts are critical in a wide range of industries. Electropolishing bestows a multitude of benefits. It is the removal of surface microscopically rough features (micro-crevices) and imperfections in stainless steel components that tend to trap contaminants. This makes electropolishing critical in the pharmaceutical and food processing industries. Smooth, polished surfaces decrease the probability of particles adhering to surfaces and assist in meeting strict contaminant standards, which increases hygiene.
The aerospace industry benefits significantly from electropolishing as well. Esthetically smooth, polished surfaces decreases drag and increases the aerodynamic efficiency of components due to lower surface roughness. In addition, the processes dependably decreases the probability of stress cracks to guarantee the longevity and dependability of key components under extreme pressure conditions.
The manufacture of medical devices heavily depends on electropolishing because of its ability to produce accurate and smooth surfaces on surgical instruments, implants, and other equipment. One recent research showed that components with electropolished surfaces can be safer for prolonged use in parts of surgery enduring environments as they improved corrosion resistance by up to 30%. Also, it improves biocompatibility which enhances patient safety and outcomes in healthcare.
Finally, electropolishing enhances the wear resistance and lowers the friction in the industrial and automotive sectors. This increases the service life of the parts during the harsh operational environments. The smooth, reflective finish offers aid not only to functionality but also to aesthetics, which is important in high-end consumer products.
How Does Electropolishing Compare to Other Stainless Steel Finishing Processes?

Electropolishing is distinctive from other stainless steel finishing processes due to its ability to achieve unique smoothness and cleanliness. Unlike mechanical polishing, which is bound to add some features to the surface, electropolishing takes a uniform layer of material away on a microscopic scale, obliterating and improving the contaminant removal and corrosion resistance. Also, it gives out a bright, reflective finish without needing extra steps, thus processing steps can be minimized. While passivation promotes corrosion resistance, it focuses exclusively on that. Electropolishing, on the other hand, simultaneously polishes, deburrs, and cleans the surface, making it optimal for applications where precision and durability are essential.
Electropolishing vs. Mechanical Polishing for Stainless Steel Parts
Stainless steel parts can be finished using either electropolishing or mechanical polishing, and the two methods differ greatly in their benefits. Electropolishing uses an electrochemical technique to strip material off the surface and achieves a very smooth, polished, passivated, and reflective surface. This method is very useful for the parameters of cleanliness and friction because it helps get rid of microscopic peaks and valleys while improving corrosion resistance as well. Studies show that in medical, pharmaceutical, and food processing industries that need hygiene, surfaces that are electropolished are able to cut the skill of bacterial adhesion by close to fifty percent.
In contrast, other techniques, like smoothing out surfaces through buffing and grinding to achieve a finer surface finish, are referred to as mechanical polishing. This is just one part of the technique because when smoothing it on, it can leave micro-abrasions and other remnants embedded, which offsets the value of the material when trying to resist corrosion. Moreover, there are other additional procedures like passivation to endorse a comparative degree of corrosion resistance, like the lack of corrosion protection that is offered by electropolishing.
Electropolishing has a lower time and cost expenditure in complex geometries and hard-to-reach areas due to the electrochemical processes treating the whole surface without direct contact, while mechanical polishing is limited by the intricacy of the design and may yield different levels of detail on the surface. Considering practical benefits alongside performance data, industries demanding high precision, cleanliness, and ruggedness rely on electropolishing as the preferred method.
Differences Between Electropolishing and Passivation of Stainless
Each method differs in cleaning stainless steel and improves its resistance using passivation and surface treatment methods of electrolytic polishing.
Electropolishing is an electrochemical cleaning method that smoothens out the surface of stainless steel. Like other polishing methods, electropolishing is aimed at improving the aesthetic and functional properties of the surface material. Thus, one of the advantages of electropolishing is surface defects such as cracks and fissures that harbor germs and corrosion. Detented surfaces electropolished with are roughness average of Ra 0.1om μm, make them very attractive for the pharmacy medicine and semiconductor industry.
The removal does not change the topology of the upper layers of the steel surface, unlike chemically polishing the nitric or citric acid. The process pumps up corrosion-resistant alloyed passivating oxide layers that not only react to regenerate the spent passivation film but also protect components, unused or unused products, depositing chromium oxide alloy. The strongest surface tie passivation removes alloyed plain free iron. Particularly, is properly done rough surface of stainless steel does not have free iron passivate at the structural surface. Electropolishing does not give ultra polish unlock key subishi’s.
Research shows that electropolished stainless steel tends to resist corrosion better than passivated surfaces do. This is especially true in harsh or corrosive environments. For example, in salt spray tests, electropolished samples had a higher rusting threshold than passivated-only surfaces. Moreover, now electropolished surfaces also have lower friction and are more easily cleaned because of their smoother surface. This property is important in industries where cleanliness and hygiene are vital.
In summary, the decision to use either electropolishing or passivation is determined by the surface finish preference, service life, resistance to corrosion, and the operating environment. Passivation works well for cost-effective applications that require basic protection. However, for critical operations that require attention to detail and high functionality, electropolishing is the better choice.
What Are the Key Steps in the Electropolishing Process?
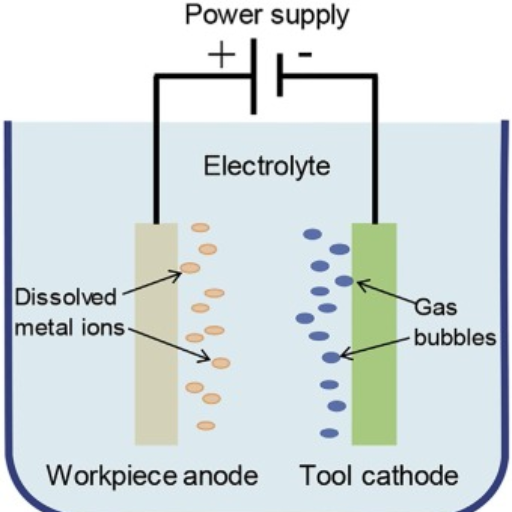
The advanced steps in the electropolishing process are:
- Preparation and Cleaning: All contaminants, including dirt and oil, are removed from the surface of the metal component.
- Immersion: The component is fully dipped in through a blended acid electrolyte tank.
- Application of Current: Electric current is supplied with the help of electricity and is responsible for a series of chemical oxidational reactions as well as physical entity dissolving from the surface of the component.
- Rinsing: the leftover products from the polishing procedure, along with electrolyte solution, are purified.
- Drying and Inspection: After achieving desired surface results the part was dried and inspected: is also considered as the final stage.
These processes combined create an incredibly sleek and refined finish.
Preparation of Stainless Steel Parts for Electropolishing
Achieving optimal results in the electropolishing of stainless steel components begins with proper preparation. Preparation activities make certain that the process removes all surface flaws to improve the part’s corrosion resistance, visual appeal, and cleanliness.
- Degreasing and Cleaning: Starting with stainless steel parts, the sharp increase in effectiveness of electropolishing requires cleaning thorough enough to eliminate oils, grease and dirt. Contaminants on the surface may lead to an unoptimized processing step such as polishing, resulting in sub-optimal results. Alkaline based degreasers work as the most efficient cleaning solutions.
- Surface Inspection: Parts must be analyzed before proceeding to the next set of instructions. An electropolished finish is greatly impacted by deep scratches, weld spatter, and pits as they greatly affect the uniformity of the part. None of the remaining surface defects apart from those addressed by means of polishing may require grinding or buffing prior to this step.
- Rinsing: A critical step in the rinsing process is where the entire surface is cleaned with deionized or purified water. Ensuring removal of cleaning agent residues, this step prevents contamination throughout the process.
- Anodic Masking (If Needed): Electropolishing can be ensured through the application of certain specified masking materials, such as specialized tapes or coatings, which prevent polishing from occurring on selected areas.
- Preliminary Testing: Evaluation of critical component requirements, such as solution concentration, polishing time, and current density, is best solved through performing test runs prior for devising a more streamlined electropolishing process.
New strides in surface preparation and monitoring techniques have enhanced reliability outcomes. Evidence suggests that proper preparation can improve corrosion resistance in stainless steel by 30%, as well as reduce biofilm adhesion in medical or food-grade applications dramatically. Following strict preparation steps, the electropolishing process achieves the desired finish to stainless steel parts from a functional and visual perspective, surpassing all requirements.
Using Electropolishing Equipment: Best Practices
Best practices suggested by experts in the industry should be followed to maximize achievement goals during the electropolishing process. *Initially** ensure that all stainless steel parts are scrubbed effectively and devoid of contaminants such as oil, scaler, grease, or any surface residues. Cleaning these items is performed through scrubbing with an appropriate detergent followed by rinsing with deionized water for minimum impurities.
In the context of polishing processes, the orderly ratio of temperature and its associated electrolyte activity have a primary bearing on the quality of polish achieved. Uniform polish as well as properly executed micro-burr removal requires temperature to be maintained at 120 – 150 ̊F (49 – 66 ̊C) along with appropriate acid mixture (sulfuric and phosphoric acid blend). Surface finishes are proven to improve with better-controlled process conditions, enhancing process accuracy as surface quality increases by as much as 20%.
With regards to current-induced parameters of the system, the value of voltage also must be monitored and regulated. *When* setting a value for current density, within the ranges of 0.1 – 0.5 A/in², active steps must be taken to ensure equal removal of material at all areas of the surface while avoiding overheating or electrolytic solution destruction. To protect system reliability and system life, regular servicing and adjusting of the electropolishing machinery require regular reliability measures.
Achieving a flawless product relies heavily on the final rinsing and drying steps of the process. After electropolishing, a thorough rinsing with deionized water serves to both neutralize remaining acid and strip away electrolyte residues. Wet components should be dried promptly by hot air or a similar method to avoid water spot etching and contamination.
Applying these practices, manufacturers are capable of achieving a consistent stainless steel surface finish, enhanced corrosion resistance, and improved multifunctionality across a wide range of industrial applications.
Quality Control in the Electrochemical Polishing Process
Applying proper quality control measures in the electrochemical polishing procedure guarantees reliability and uniform results within industrial applications. Some important aspects to control are: the composition and the temperature of the electrolyte, current density, and processing time. For instance, electrolyte composition analysis is critical in maintaining the proper chemical balance, as improper concentration may lead to surface defects or diminished resistance to corrosion. Other studies state that current density between 10 to 40 A/dm² is ideal for most cases in achieving defect-free, smooth surfaces, however, this may differ depending on the material and finish.
Equally important is the control of temperature; an allowance outside the recommended boundaries may impact the effectiveness of the polishing cycle. For stainless steel, the electrolyte temperature typically varies from 110°F (43°C) to 180°F (82°C), this ensures that material is removed properly while not damaging the substrate. Additionally, the midpoints of precise defined criteria, such as for the desired surface finish, need to be followed in order to prevent excessive polishing which adversely impacts the structural integrity of the components.
Use of optical and scanning electron microscopes cuts QC verification for surface smoothness and imperfections to a new level. Moreover, periodic verification of electrochemical polishing effectiveness over time with corrosion resistance salt spray or humidity tests ensures the treatment lasts. Implementing and monitoring such controls allow the industries to provide components with performance as well as durability beyond industry benchmarks.
How Does Electropolishing Affect Different Stainless Steel Alloys?

Effects of Electropolishing on Different Stainless Steel Alloys
Electropolishing improves the surface finish and corrosion resistance of numerous alloys of stainless steel, including complex austenitic stainless steels 304 and 316. These grades are smooth and shiny with durable surfaces because of the high chromium and nickel content, and their resistance to corrosion. Duplex stainless steels are also electropolished, which improves their resistance to pitting and stress corrosion cracking. Ferritic and martensitic stainless steels do experience some improvement, but undergo lower grades of corrosion resistance compared to austenitic, which renders them less effective for this process. The right alloy has to be picked to obtain maximum efficacy from electropolishing.
Electropolishing Results on Common Stainless Steel Alloys
The results of electropolishing are different depending on the stainless steel alloy being processed. In the case of austenitic stainless steels like 304 and 316 grades, surface roughness is decreased significantly, with Ra values reaching 0.1–0.2 µm, which improves aesthetics and clean. After electropolishing, these alloys still retain their excellent corrosion resistance and mechanical properties, which makes them suited for hygienic and high-purity applications.
Duplex stainless steels, having more balanced mechanical strength and corrosion resistance, also reap positive benefits. Electropolishing improves their resistance to pitting and crevice corrosion, particularly in maritime and chloride-rich environments where harsh conditions require robust durability.
For ferritic stainless steels, improvements are not as significant due to a lower composition of nickel and chromium relative to austenitic grades. There are also improvements in surface smoothness and aesthetics, but the corrosion resistance change is minimal. Martensitic stainless steels are similarly improved in surface finish and contamination, but need to be more tightly controlled during processes to avoid hydrogen embrittlement for highly mechanically stressed applications.
Ultimately, the effectiveness of electropolishing processes is a function of choosing the proper alloy for a particular application. The environment in which the electropolished part operates and certain properties of the part, such as corrosion resistance, mechanical strength, or appearance, should be taken into account when selecting materials for electropolishing.
How Electropolishing Improves Weld Appearances
The use of electropolishing adds tremendous cosmetic value to welding through surface finishing, especially smoothing and brightening the surface. As a whole, welded sections tend to have contour irregularities such as heat-affected zones, which tend to trivialize the aesthetic and performance value of the material. To remove these imperfections, which often exist in welds, the process of polishing data defects by removing a layer of metal works in restoring the finish, reflectivity, and smoothness, making the weld more appealing.
Electropolishing processes are actually able to provide even better surface enhancement by mechanically cleaning an area; this helps remove contaminants like embedded oxides or residual particles. Studies have shown that polishing significantly improves surface roughness, and in some cases, reductions of Ra measurements by 50% or more were observed. If essential, these are critical in the spheres of high-hygiene food processing or pharmaceutical manufacturing. Thus, why do these benefits arise? The answer is simple: most of the bacterial niches are covered, which creates smooth, easy surfaces.
At the finish, polish improves the apparent functionality, enhances ease of cleaning, and aesthetic appeal of welds. Due to the process also capturing fields of chromium at the surface of the stainless steel, polishing enables the application of protectorate coatings, which increases durability and resistance against the effects of corrosion; essential in harsh surroundings. All of these aspects combined indicate the requirement of polished steel after the critical applications to make it truly multifunctional.
What Industries Benefit Most from Electropolished Stainless Steel?

Let us take a look at the main beneficiaries of electropolished stainless steel:
- Pharmaceutical and Biotechnology: Stainless steel is used for sanitary surfaces in which cleanliness and contamination control need to be maintained.
- Food and Beverage: Stainless steel is helpful for ensuring safety and hygienic processing environments as well.
- Medical and Healthcare: Sterilization yields improvement in the cleanliness and durability of surgical instruments and other medical devices.
- Semiconductor: Manufacturing processes require ultra-smooth surfaces with precision.
- Aerospace: Critical components undergo performance and corrosion resistance improvement.
These industries need polished stainless steel because it provides its users with great surfaces and polish. It is clean, durable, and scratch resistant.
Aerospace Applications for Electropolished Stainless Steel
Due to meeting high standards of performance and durability, electropolished stainless steel has important applications in the aerospace industry. Given below are the benefits and applications.
- Enhanced Corrosion Resistance: The polish provides a smooth and passive oxide surface coating that protects the polished material from corrosion. This is critical for aerospace components that undergo extreme conditions at high altitudes.
- Weight Reduction: This technique permits the construction of lighter parts, which increases fuel efficiency and the overall performance of the aircraft.
- Improved Fatigue Strength: Electropolishing enhances the fatigue life of critical components like turbine blades and structural components by alleviating stress concentrations caused by micro-scratches, pits, and spurious surface blemishes.
- Aerodynamic Efficiency: Optimum performance of any aerodynamic structure is achieved by the reduction of air resistance which results in increased fuel efficiency. This is achieved due to ultra-smooth and clean surfaces.
- Precision and Reliability: The performance of hydraulic systems and fasteners, along with their intricate component, is reliable due to accurate dimensions and thorough electropolishing.
- Compliance with Standards: Stainless steel components that are aerospace grade undergo electropolishing due to very stringent industry regulations regarding the degree of cleanness and cleanliness defined by AMS and AS9100 standards.
The features of electropolished stainless steel make it an essential material in the aerospace sector as it enables manufacturers to maintain safety, efficiency, and reliability in aircraft and spacecraft operations.
Medical Industry Standards for Electropolishing Stainless Steel
Electropolishing enables stainless steel parts in the medical field to pass strict cleanliness, safety, and functionality benchmarks. Compliance to ASTM B912 and ISO 13485 provides standards to measure if electropolishing is done adequately and consistently. The electropolishing process eliminates microscopic peaks and surface contaminants, producing a smooth surface that is corrosion-free and resists bacterial growth.
Electropolishing enhances biocompatibility of surgical instruments, implantable devices, and diagnostic stainless steel tools by reducing surface roughness to 20 Ra (μin) or lower, depending on usage. In addition, research suggests that due to greater exposure to the wearing out of cycles of forced cleaning and the polishing of the device, medical equipment’s endurance and lifespan is greatly improved with polishing.
Compliance with medical standards in manufacturing is essential not only for meeting regulations, but also for assuring safety and trust in place for users. Quality checks in audits pose a challenge, whether processes such as polishing undergo sound management. The technical discipline of polishing for the medical field paves the way for the strict benchmarks that ensure the safety and quality of devices before they are provided to patients.
Food Processing Equipment and Electropolishing
Electropolishing is essential in the food processing industry as it improves the efficiency, cleanliness, and lifespan of equipment. This process smoothens the surface at the microscopic level and reduces the chances of food and bacteria harboring in various “nooks and crannies”. Electropolishing also polishes stainless steel equipment to a better state than the corrosion resistance it can offer due to exposure to cleaning chemicals and heavy-duty sanitization procedures.
In comparison to mechanically polished surfaces, industry standards now consider electropolished surfaces to outperform them in hygiene. A case in point would be that electropolished surfaces are reported to have reduced microbial adhesion by 50%, which makes for enhanced food safety in processing environments. In addition, with electropolishing, the chances of contamination by FDA and EU standards for food-grade equipment are enhanced due to these bodies being far less likely to be breached. With electropolishing, machinery withstands the demand for high sanitation and hygiene maintenance while manufacturers ensure improved machinery durability.
Reference Sources
- High-Voltage Electropolishing (HVEP) of AISI 304L Stainless Steel1:
- Key Findings: This study introduced HVEP as a novel method to enhance the surface properties of AISI 304L stainless steel. The process incorporated copper ions into the passive surface layer, improving bactericidal properties while reducing carcinogenic chromium VI content. Higher voltages increased copper incorporation and altered the chromium-to-iron ratio.
- Methodology: The process used phosphoric acid and copper nitrate as electrolytes, with voltages ranging from 14V to 450V. X-ray Photoelectron Spectroscopy (XPS) was employed to analyze the passive layer’s composition.
- Effects of Electropolishing Parameters on Corrosion Resistance of 316L Stainless Steel2:
- Key Findings: The study demonstrated that electropolishing significantly improved the corrosion resistance of 316L stainless steel. Optimal parameters included a 5mm electrode gap and a 5-minute polishing time, which enhanced surface smoothness and reduced localized corrosion.
- Methodology: The research utilized linear polarization and electrochemical potentiokinetic repassivation (EPR) tests to evaluate corrosion resistance. Surface roughness and passive film composition were analyzed using XPS and Auger Electron Spectroscopy (AES).
- Innovative Electropolishing Processes for Medical Devices3:
- Key Findings: This paper focused on electropolishing techniques tailored for medical-grade stainless steel. It highlighted the process’s ability to enhance biocompatibility, corrosion resistance, and surface smoothness, critical for medical applications.
- Methodology: The study reviewed various electropolishing methods and their impact on surface characteristics, emphasizing the importance of process optimization for medical device manufacturing.
Frequently Asked Questions (FAQs)
Q: How does electropolishing work?
A: Electropolishing is an electrochemical process that removes the outer layer of metal using an electrical current combined with a chemical bath, typically containing sulfuric acid. When the electrical current is applied, metal ions on the surface dissolve, removing microscopic peaks and irregularities. This process, also known as electrochemical polishing, works by preferentially dissolving raised areas at a faster rate than recessed areas, resulting in a smooth, bright finish. Unlike mechanical polishing, electropolishing removes material at a molecular level, creating a truly uniform surface without creating new stress points.
Q: Why should I electropolish stainless steel?
A: Electropolishing stainless steel offers numerous benefits, including enhanced corrosion resistance, improved cleanability, reduced product contamination, and a bright, aesthetically pleasing appearance. The process removes free iron and other contaminants from the surface while enhancing the chromium-rich passive layer. Additionally, electropolishing can help eliminate microscopic surface defects that could harbor bacteria or initiate corrosion. Many industries, from medical device manufacturing to food processing, rely on electropolished stainless steel or similar alloys to meet strict cleanliness and durability requirements.
Q: What are the process steps for electropolishing stainless steel?
A: The process of electropolishing typically follows these steps: 1) Pre-cleaning to remove oils, greases, and contaminants; 2) Racking the parts to ensure proper electrical contact; 3) Immersion in the electropolishing solution (typically containing phosphoric and sulfuric acid); 4) Application of direct current with the workpiece as the anode; 5) Removal and rinsing to neutralize any remaining acids; 6) Passivation treatment to enhance the chromium oxide layer; and 7) Final inspection to ensure the parts meet the industry standard specification requirements. The electropolishing process may vary slightly depending on the specific application and metal composition.
Q: How does electropolishing improve the surface of stainless steel?
A: Electropolishing improves the surface of stainless steel by removing a microscopic layer of metal, including impurities, embedded particles, and surface defects. This creates a smoother surface with significantly reduced roughness values. The process also increases the chromium-to-iron ratio at the surface, enhancing the passive layer that gives stainless steel its corrosion resistance. Additionally, electropolishing eliminates the stressed and smeared metal layer created by mechanical processing, leaving a surface that is not only visually appealing but also functionally superior with improved fatigue resistance and reduced potential for contamination trapping.
Q: What is the difference between mechanical polishing and electropolishing?
A: While both methods improve surface finish, mechanical polishing and electropolishing work very differently. Mechanical polishing uses abrasives to physically remove material and smooth the metal surface, which can create smeared metal, embedded abrasives, and microscopic scratches. It may also introduce stress that can lead to premature failure. Electropolishing, by contrast, is an electrochemical process that selectively dissolves the outer layer of metal, producing a truly smooth surface free of embedded particles or directional lines. Electropolishing also enhances corrosion resistance through passivation, removes burrs, and provides a more consistent finish than machine polishing alone can achieve.
Q: What industries commonly use electropolishing for stainless steel?
A: Electropolishing is widely used across numerous industries, including medical device manufacturing, pharmaceutical processing, semiconductor fabrication, food and beverage production, aerospace, nuclear, and chemical processing. Companies like New England Electropolishing specialize in providing this service to industries with strict requirements. The process is particularly valuable wherever cleanliness, corrosion resistance, or aesthetic appearance are critical. Any application where bacteria prevention, easy sterilization, or resistance to product buildup is needed can benefit from electropolishing. The process helps manufacturers meet regulatory requirements and extend the service life of stainless steel components.
Q: How do I know if my metal surface needs electropolishing?
A: Your metal surface may benefit from electropolishing if you’re experiencing issues with corrosion, product contamination, difficult cleaning, or inconsistent appearance. If your application requires ultra-clean surfaces, such as for medical implants or pharmaceutical processing equipment, electropolishing should be considered. Parts with complex geometries that are difficult to polish mechanically are also good candidates. Looking at images of electropolishing results can help you understand the improvement possible. Many manufacturers also specify electropolishing when parts need to meet strict surface roughness requirements or when weld areas need to be blended without dimensional changes.
Q: Can electropolishing replace the need to electroplate stainless steel?
A: Yes, in many applications, electropolishing can eliminate the need to electroplate stainless steel. While electroplating adds a layer of material to the surface, electropolishing removes material to create a naturally lustrous finish with enhanced properties inherent to the base metal. Electropolishing provides superior corrosion resistance by enhancing the natural passive layer of stainless steel rather than adding a potentially incompatible coating that might chip or peel. For applications requiring both aesthetic appeal and performance benefits, electropolishing is often preferred over electroplating, particularly for medical, food processing, and pharmaceutical equipment where material compliance and longevity are critical.